How To Get Magnetic Quantum Number May 14 2023 nbsp 0183 32 The magnetic quantum number m l together with the principal n azimuthal l and spin m s quantum numbers is one of four quantum numbers used in atomic physics to characterize the special quantum state of an electron
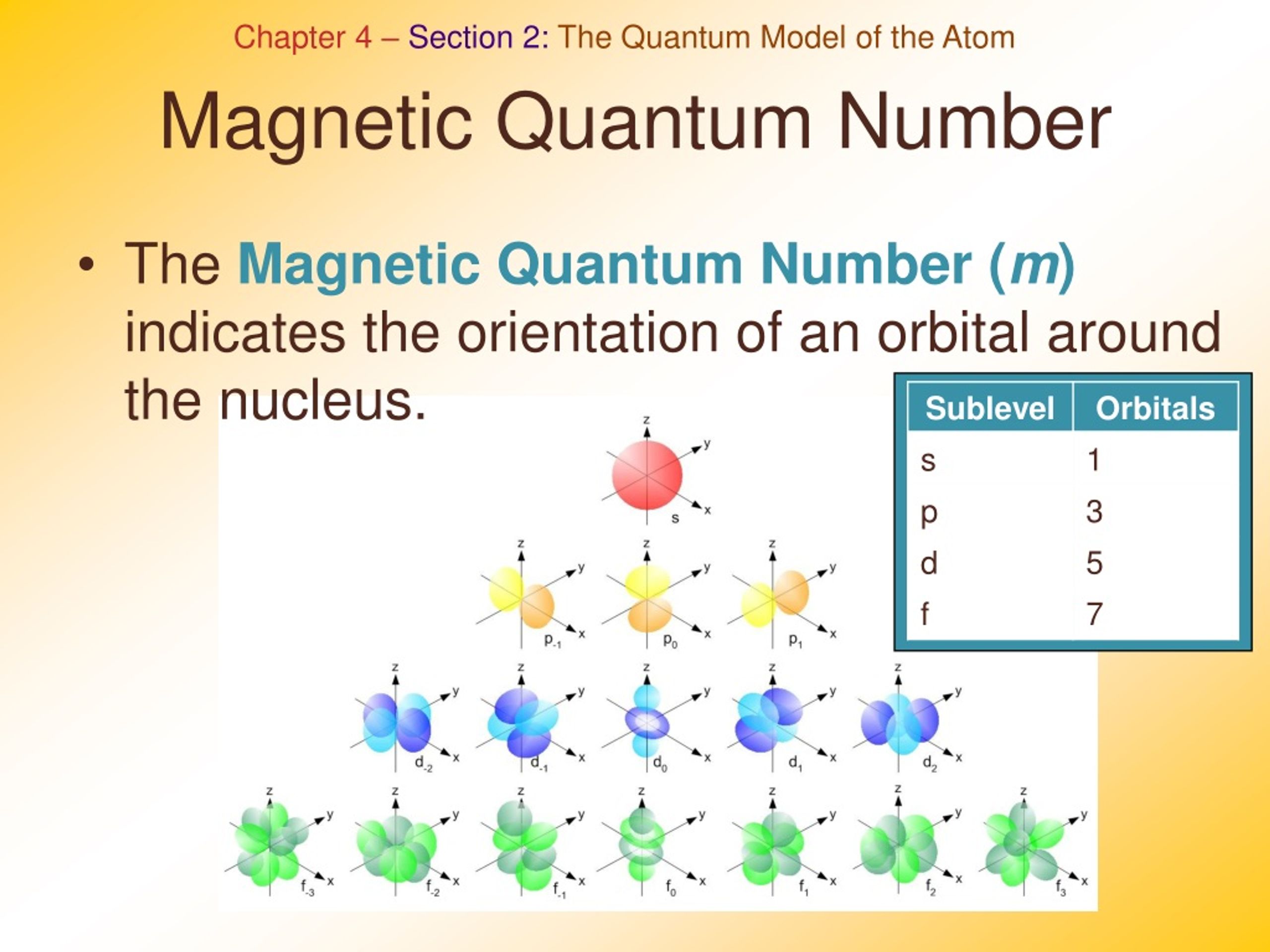
The value ml is the magnetic quantum number The possible ml values follow the equation l 1 2 3 l The ml s values represent the orbitals in a subshell which actually contain electrons Apr 24 2017 nbsp 0183 32 Call the second quantum number l Represent the magnetic quantum number that denotes orientation of the electron in space by l to l For for the case of sodium that could be 2 1 0 1 and 2 if the second quantum number was 2 Consider the rotation of
How To Get Magnetic Quantum Number
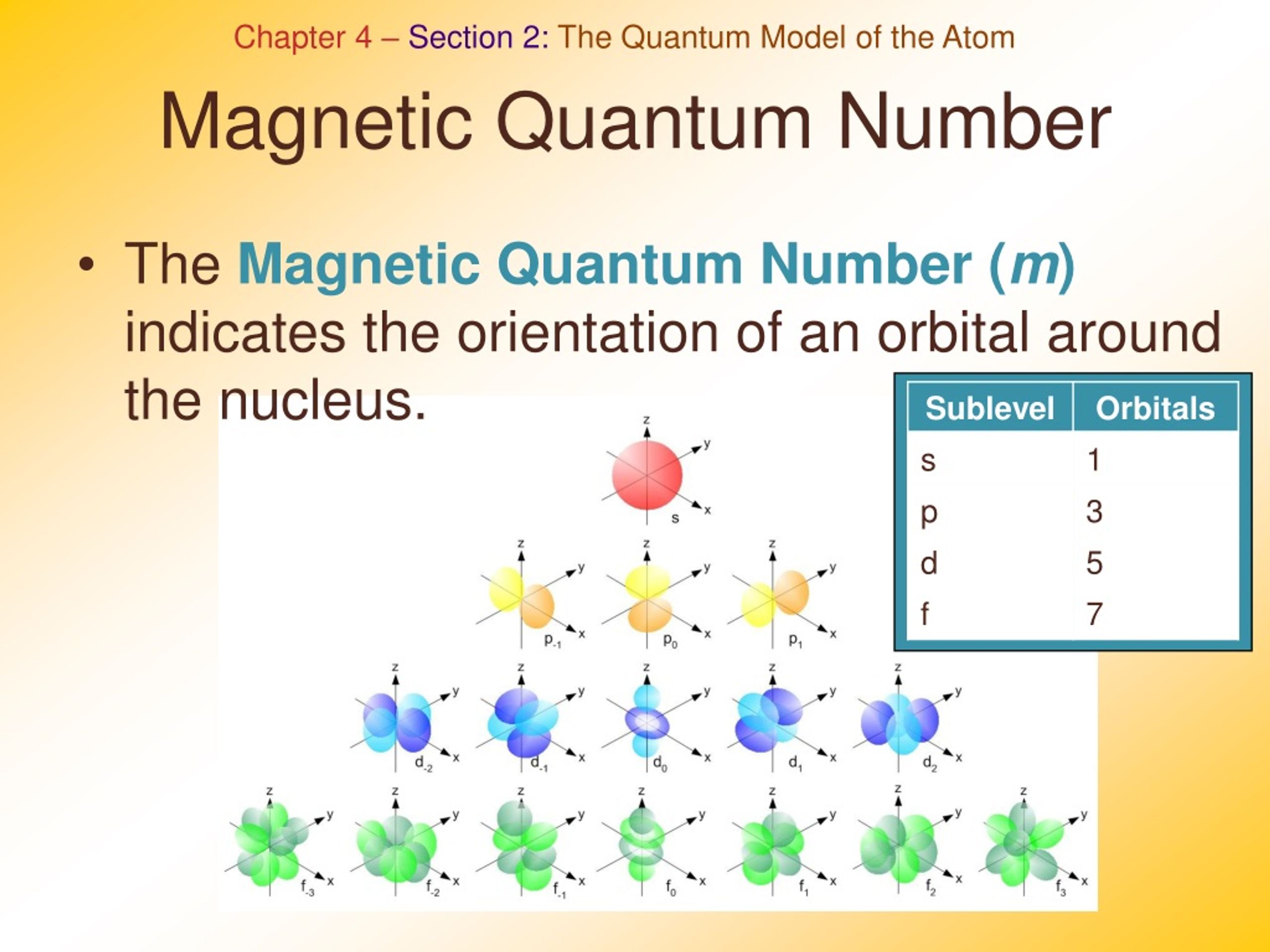 How To Get Magnetic Quantum Number
How To Get Magnetic Quantum Number
https://image4.slideserve.com/298336/magnetic-quantum-number-l.jpg
Feb 8 2025 nbsp 0183 32 Solved Examples on Quantum Numbers Example 1 Write down all the other quantum numbers of an electron for the principal quantum number n 3 Solution Principal quantum number n 3 given The possible values of azimuthal quantum number l 0 to n 1 0 1 2 The possible values of magnetic quantum number m l to l including 0
Templates are pre-designed documents or files that can be used for different functions. They can conserve effort and time by providing a ready-made format and design for developing different sort of content. Templates can be utilized for personal or expert projects, such as resumes, invites, leaflets, newsletters, reports, discussions, and more.
How To Get Magnetic Quantum Number

Orbitals Quantum Numbers Electron Configuration Multiple Choice
Quantum Numbers Spin Quantum Number Ms Chem161 7 6 YouTube

Magnetic Quantum Number Wiki Everipedia

PPT Chapter 5 Electrons In Atoms PowerPoint Presentation Free
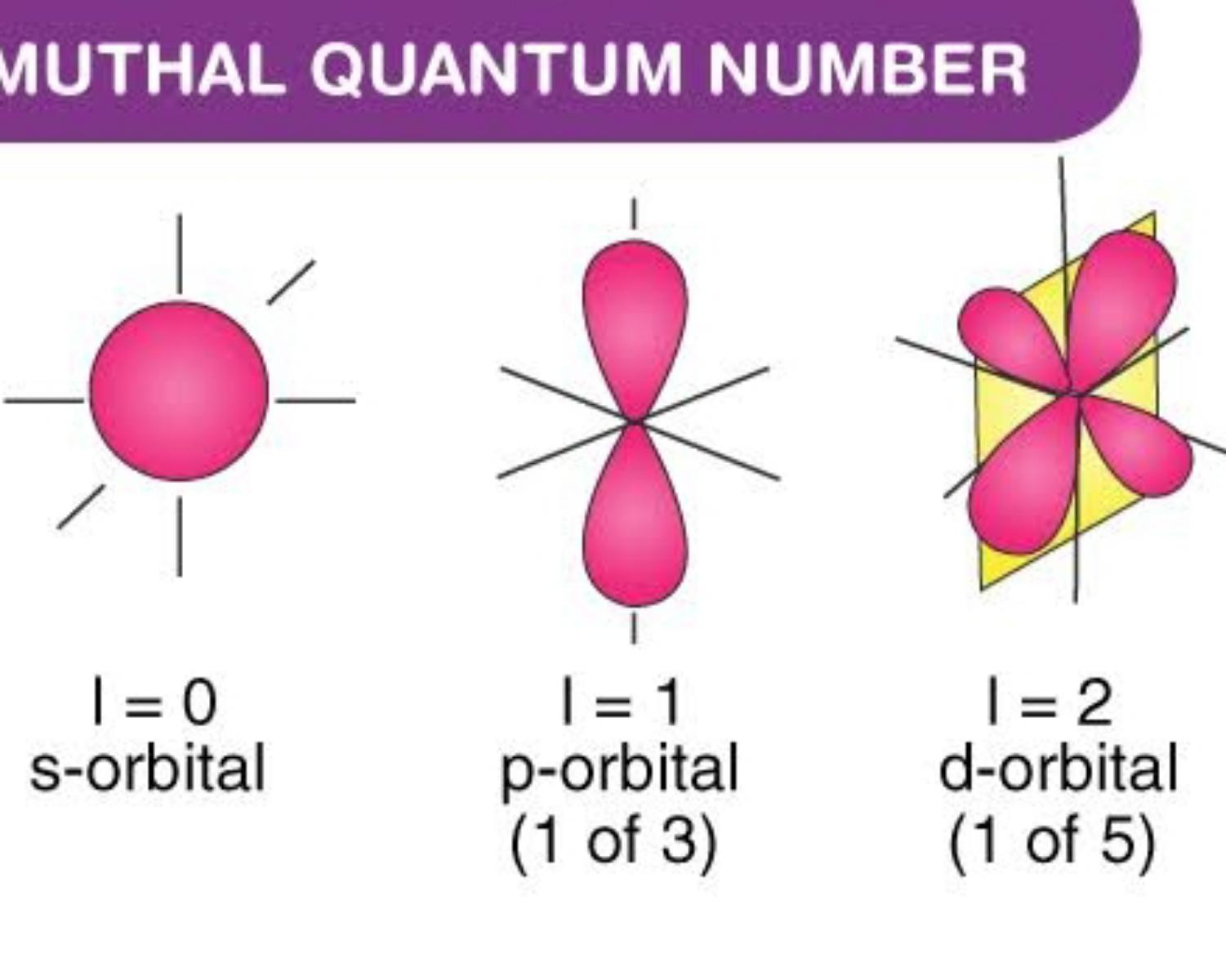
Spin Quantum Numbers ms Deepstash

Spin Quantum Number Definition Example Video Lesson Transcript
https://en.wikipedia.org › wiki › Magnetic_quantum_number
In atomic physics a magnetic quantum number is a quantum number used to distinguish quantum states of an electron or other particle according to its angular momentum along a given axis in space The orbital magnetic quantum number ml or m a distinguishes the orbitals available within a given subshell of an atom
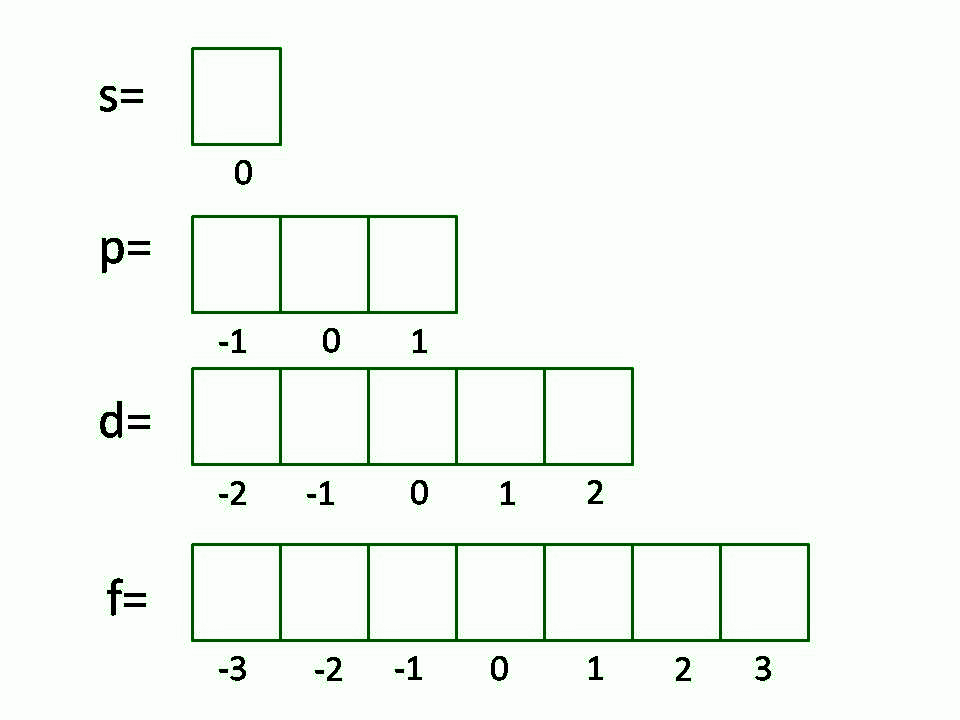
https://chem.libretexts.org › Bookshelves › Physical...
Aug 14 2024 nbsp 0183 32 The Magnetic Quantum Number m l The magnetic quantum number m l determines the number of orbitals and their orientation within a subshell Consequently its value depends on the orbital angular momentum quantum number l

https://www.chemistrylearner.com › magnetic-quantum-number.html
How to Find Magnetic Quantum Number The magnetic quantum number is denoted by the symbol m l whose values depend on the azimuthal quantum number l The formula is as follows For each value of l m l takes values ranging from l to l The total number of allowed values of m l is 2l 1 For example the l 1 represents the p subshell The

https://byjus.com › chemistry › quantum-numbers
Magnetic Quantum Number The total number of orbitals in a subshell and the orientation of these orbitals are determined by the magnetic quantum number It is denoted by the symbol m l This number yields the projection of the angular momentum corresponding to the orbital along a

https://socratic.org › questions › how-would-you...
Feb 19 2016 nbsp 0183 32 m l is the magnetic quantum number corresponding to the projection of the angular momentum of an orbital i e its orientation in space As the symbol suggests it has to do with l the angular momentum quantum number
Oct 27 2023 nbsp 0183 32 Can someone make sense of how for a given value of l azimuthal quantum number the number of magnetic quantum numbers or m subscript l magnetic quantum number ranged from l to l lowercase L in turn giving 2l 1 different m subscript l values Magnetic Quantum Number Magnetic Quantum Number denoted by the symbol m is what represents the orientation of atomic orbital in space The value of the Magnetic Quantum Number m depends on the value of l Magnetic Quantum Number can have a total number of 2l 1
Magnetic quantum number m Indicates the orientation of the orbital Spin quantum number s Tell which way the electron rotates Easy right Let s go with the important thing To get the quantum numbers you just have to follow 2 simple steps Write the electron configuration