How To Find Value Of Magnetic Quantum Number WEB n principal quantum number major energy level Values are 1 2 3 azimuthal quantum number energy sublevel Values are 0 to n 1 m magnetic quantum number the orbital in the sublevel Values are 0 m s spin quantum number electron in orbital Values are 1 2 or 1 2
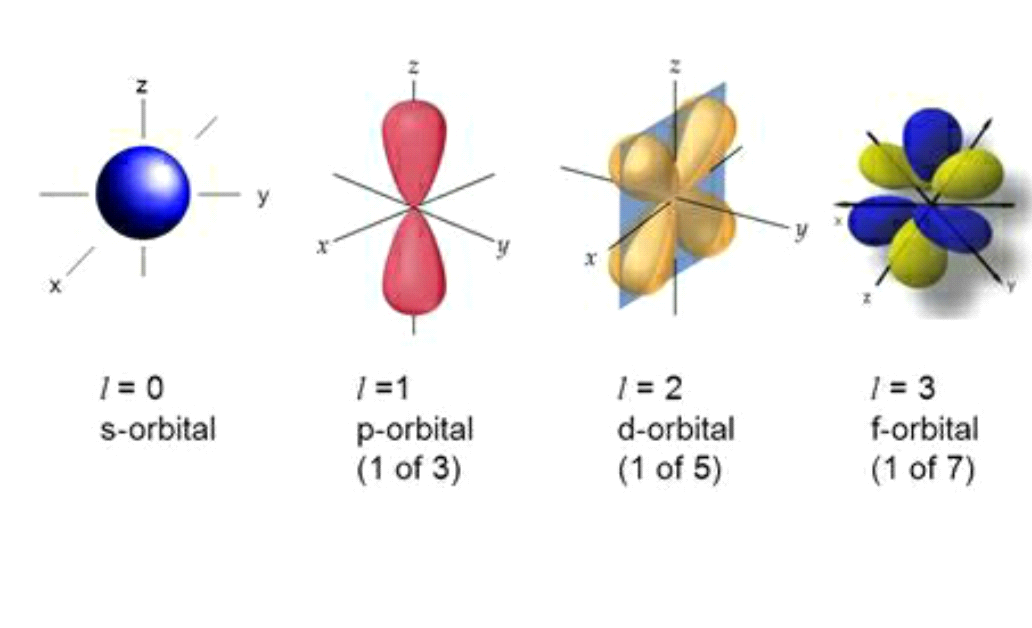
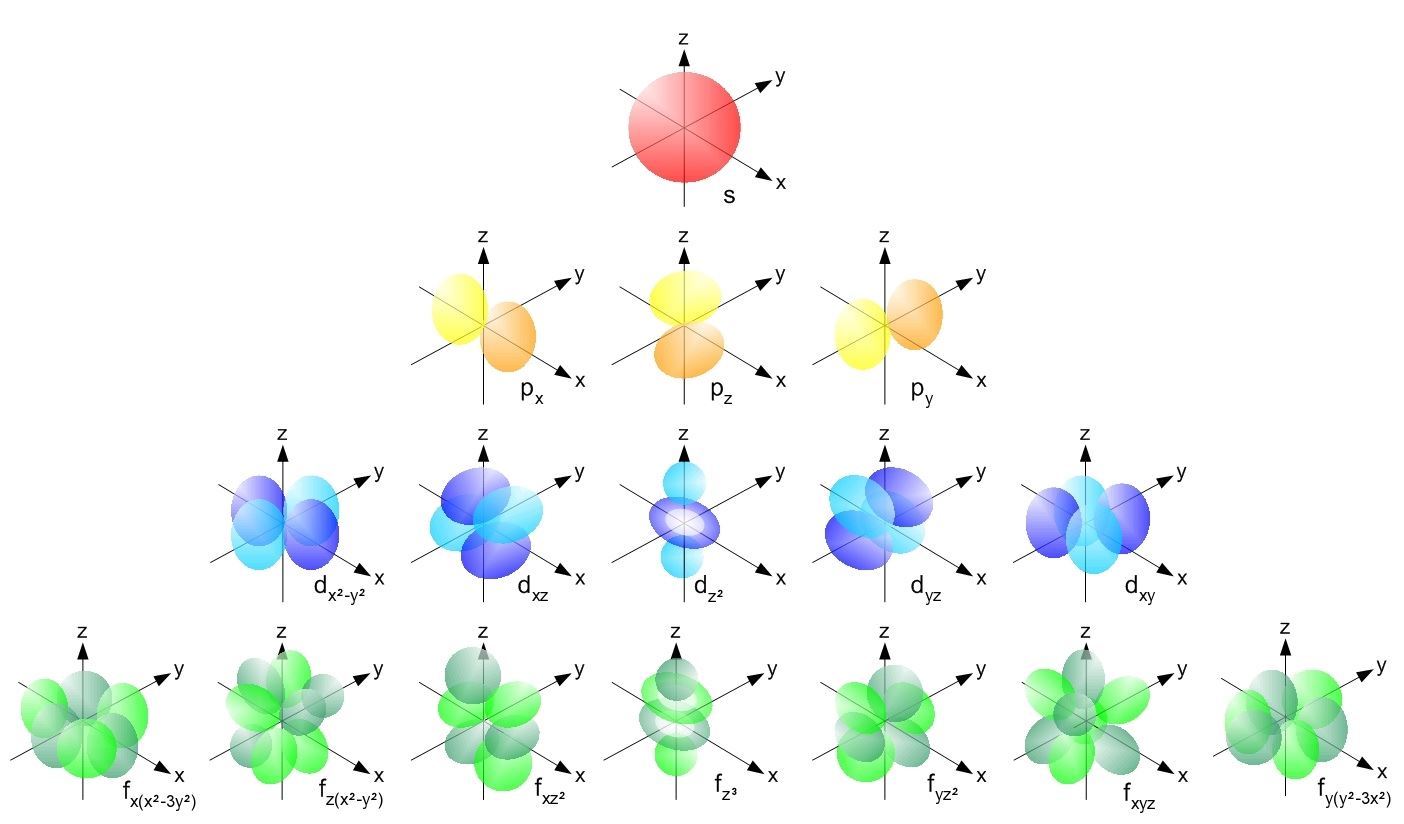
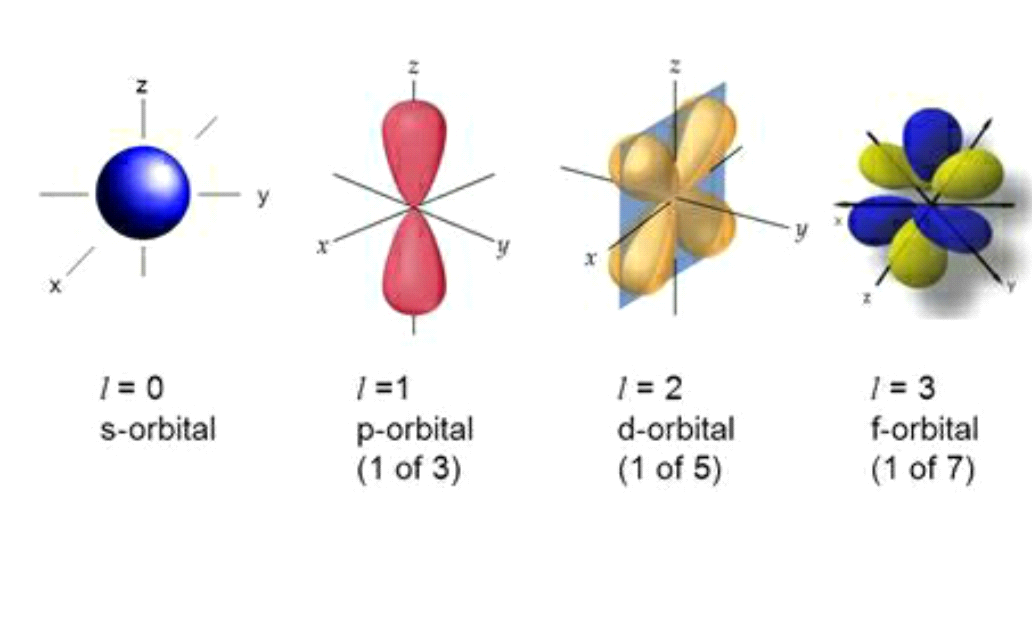
WEB Electrons in a given subshell of an atom such as s p d or f are defined by values of 0 1 2 or 3 The orbital magnetic quantum number takes integer values in the range from to including zero 3 Thus the s p d and f subshells contain 1 3 5 and 7 orbitals each WEB If l is equal to one what are the allowed values for the magnetic quantum number ml is equal to This goes from negative l to positive l so any integral value from negative l to positive l Negative l would be negative one so let s go ahead and write this in here
How To Find Value Of Magnetic Quantum Number
 How To Find Value Of Magnetic Quantum Number
How To Find Value Of Magnetic Quantum Number
https://www.w3schools.blog/wp-content/uploads/2019/08/word-image-73.png
WEB Azimuthal quantum number l 2 Magnetic quantum number ml 2 1 0 1 2 Question Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 1 2 in chromium Solution Spin can either be 1 2 or 1 2 So it is 2 electrons If one has spin 1 2 other will have spin
Templates are pre-designed documents or files that can be used for various functions. They can conserve time and effort by offering a ready-made format and layout for developing various sort of material. Templates can be utilized for personal or expert projects, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
How To Find Value Of Magnetic Quantum Number
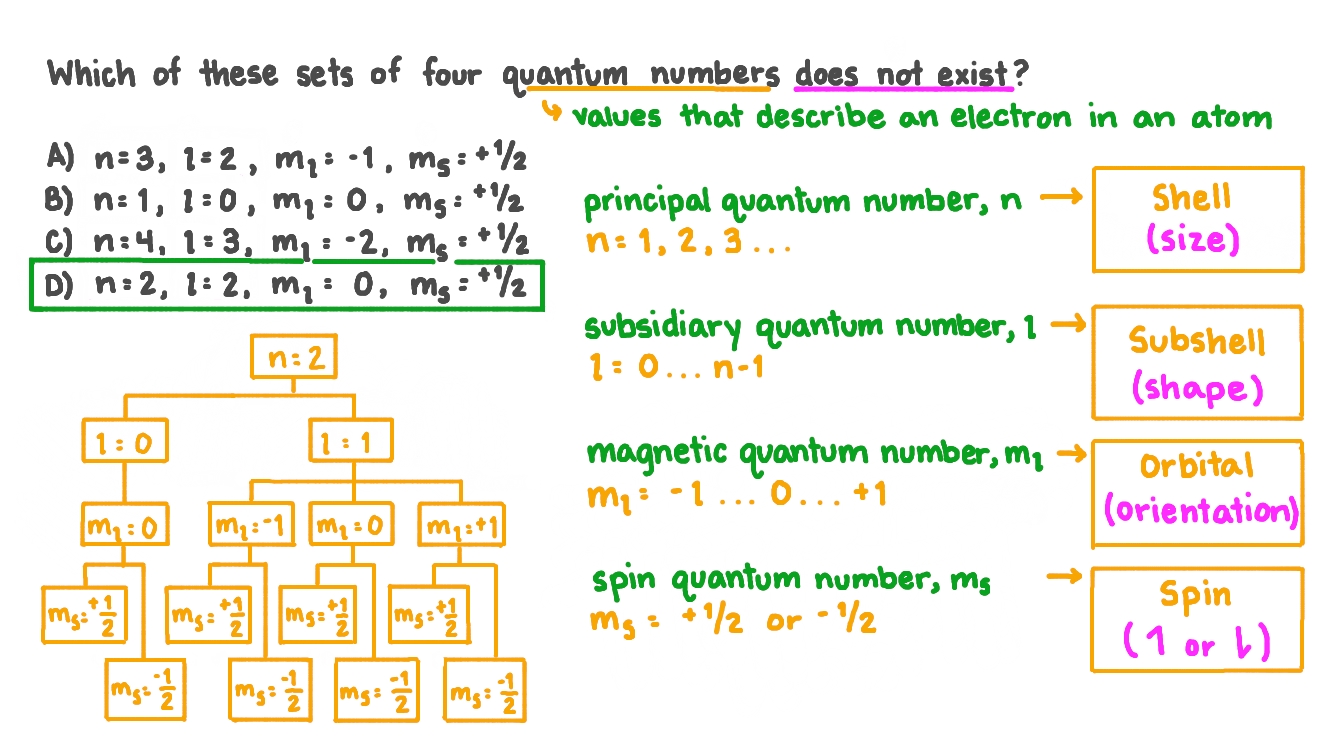
Question Video Identifying Which Set Of Quantum Numbers Does Not Exist

Quantum Numbers Magnetic Quantum Number Ml Chem161 7 6 YouTube
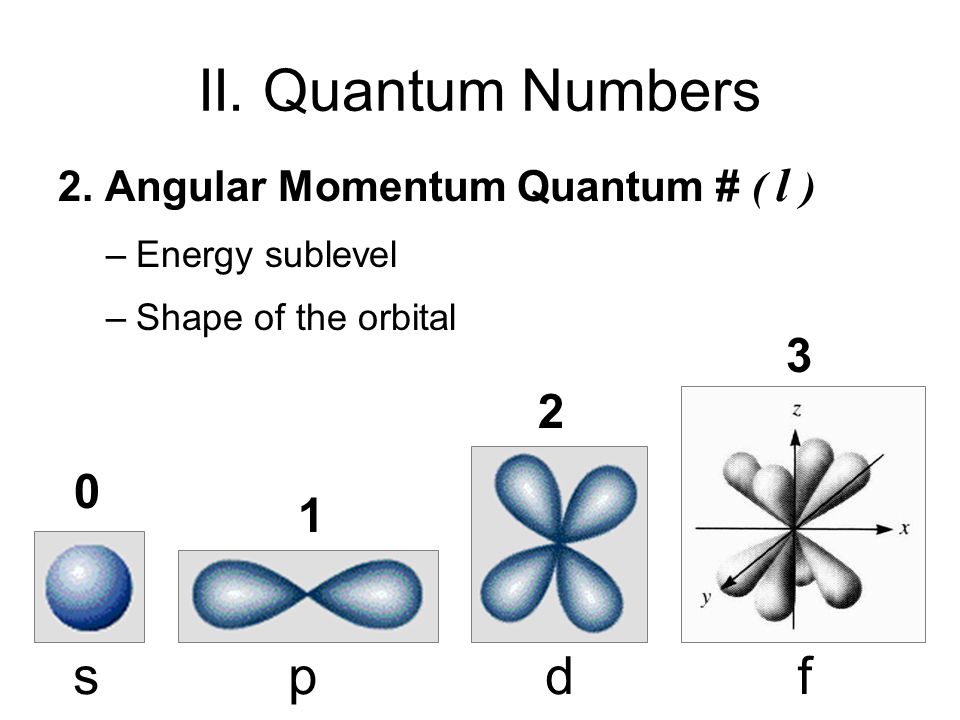
What Are The Four Quantum Numbers In Chemistry Socratic
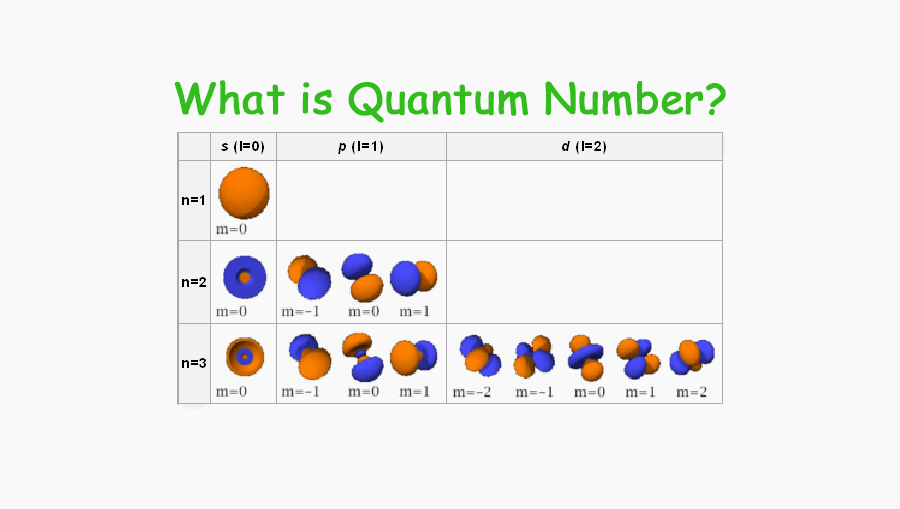
What Is Quantum Number What Do Quantum Number Determine Tuition Tube

Quantum Number Easy Science Teaching Chemistry Chemistry Education
Quantum Numbers Principal Azimuthal Magnetic And Spin Definition
https://www.chemistrylearner.com/magnetic-quantum-number.html
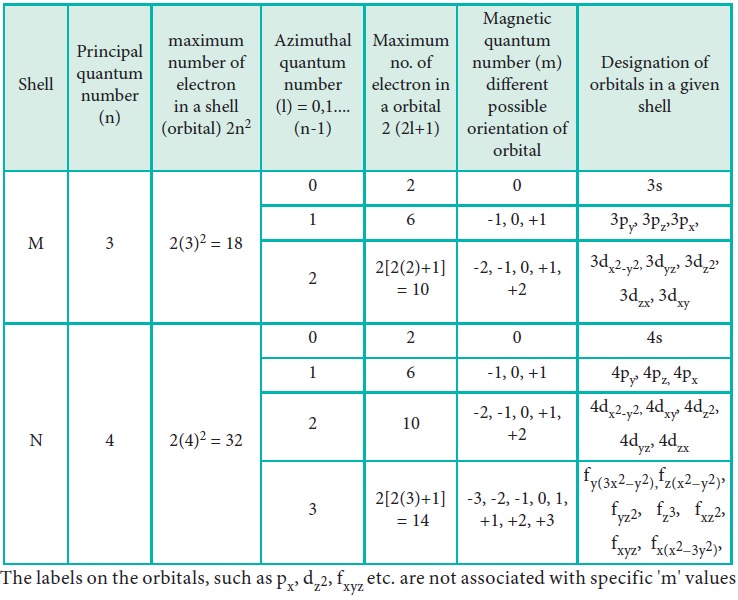
WEB How to Find Magnetic Quantum Number The magnetic quantum number is denoted by the symbol ml whose values depend on the azimuthal quantum number l The formula is as follows For each value of l ml takes values ranging from l to l The total number of allowed values of ml is 2l 1
https://chem.libretexts.org/Bookshelves/General...
WEB The value ml is the magnetic quantum number The possible ml values follow the equation l 1 2 3 l The ml s values represent the orbitals in a subshell which actually contain electrons

https://study.com/skill/learn/how-to-identify-the...
WEB Step 1 Find the element on the periodic table Step 2 Determine n and l by identifying the period and the block that the element is located in Step 3 Determine ml by labeling the block from
https://chem.libretexts.org/Courses/Oregon...
WEB The magnetic quantum number m l with 2l 1 values ranging from l to l describes the orientation of the orbital in space In addition each electron has a spin quantum number m s that can be equal to 177 dfrac 1 2

https://chem.libretexts.org/Bookshelves/Physical...
WEB Jan 30 2023 nbsp 0183 32 The Magnetic Quantum Number m l The magnetic quantum number m l determines the number of orbitals and their orientation within a subshell Consequently its value depends on the orbital angular momentum quantum number l
WEB Feb 20 2022 nbsp 0183 32 These are expressed in the form n l m l m s see Table For electrons in atoms the principal quantum number can have the values n 1 2 3 Once n is known the values of the angular momentum quantum number are limited to l 1 2 3 n 1 WEB How to find the magnetic quantum number For a given value of l m has any integral value between l to l Therefore m 0 177 1 177 2 177 3 177 l where the total number of values of m equal to 2l 1 For the s wave l 0 and m has also 1 Therefore the s orbital has only one orientation in space
WEB Physics library gt Quantum Physics gt Quantum numbers and orbitals Quantum numbers for the first four shells Google Classroom Microsoft Teams About Transcript Calculates number of orbitals and number of electrons in different kinds of orbitals for n 1 to 4