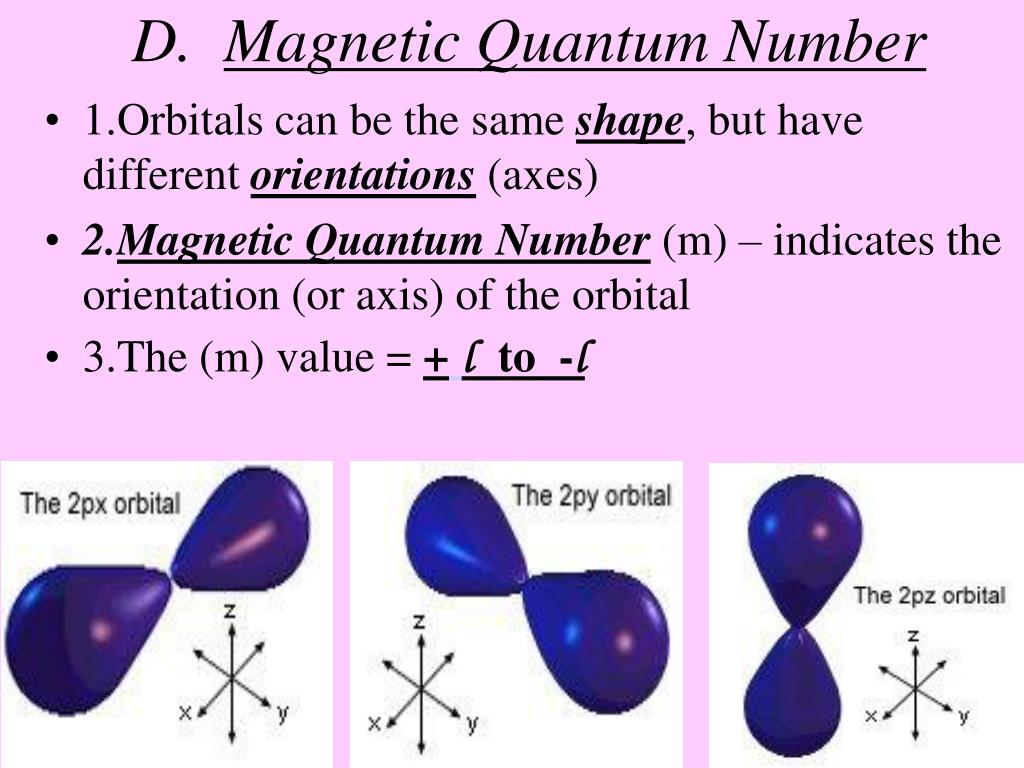
What Is Magnetic Quantum Number b The principal quantum number determines the orientation and energy of the orbital c Magnetic quantum number determines the size of the orbital d Spin quantum number of an
An electron having spin quantum number of s 1 2 and magnetic quantum number m 3 can be present in A Magnetic quantum number can have the value 0 n 1 R Magnetic quantum number specifies the number of orbitals
What Is Magnetic Quantum Number
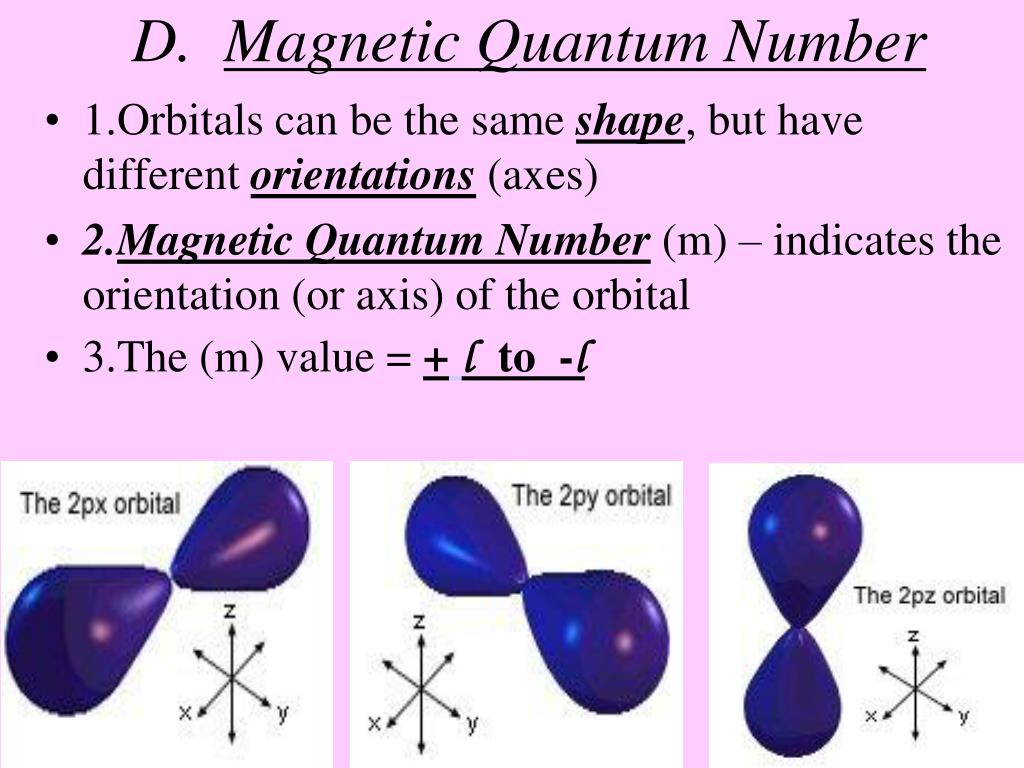 What Is Magnetic Quantum Number
What Is Magnetic Quantum Number
https://image1.slideserve.com/2012365/d-magnetic-quantum-number-l.jpg
Number of electrons of manganese Z 25 with magnetic quantum number value 0 is 13 The electronic configuration is as shown In 3d subshell there is only one electron with m 0
Pre-crafted templates use a time-saving service for producing a diverse series of files and files. These pre-designed formats and designs can be used for numerous individual and professional jobs, consisting of resumes, invites, leaflets, newsletters, reports, discussions, and more, enhancing the material development procedure.
What Is Magnetic Quantum Number
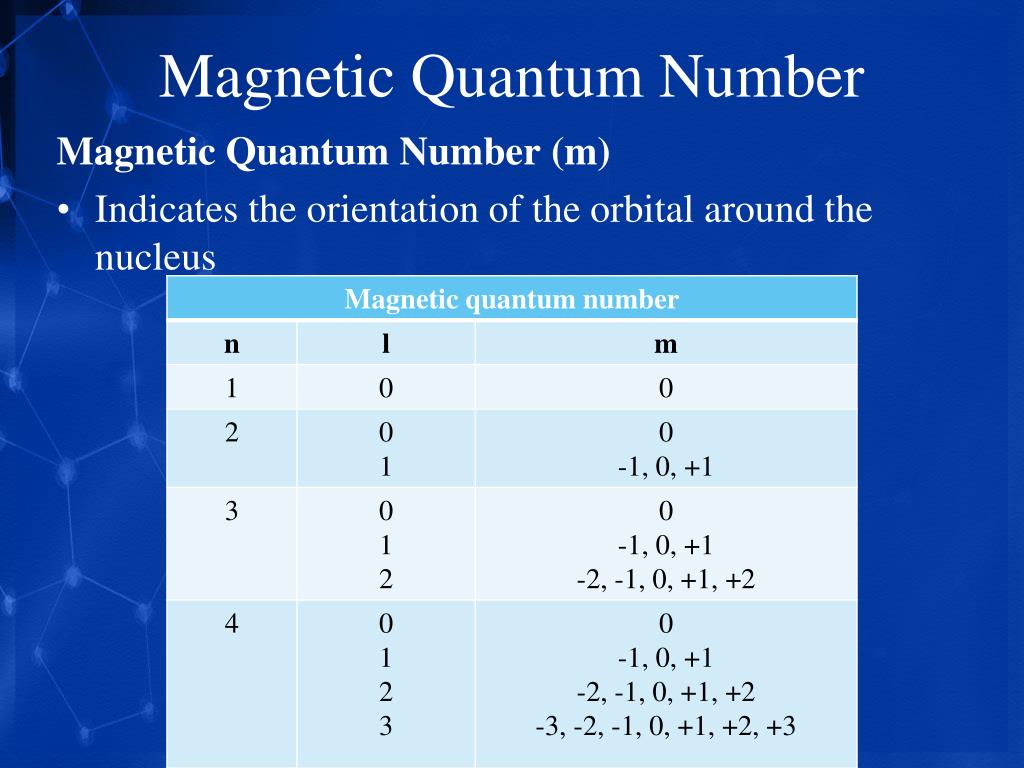
PPT Arrangement Of Electrons In Atoms PowerPoint Presentation Free
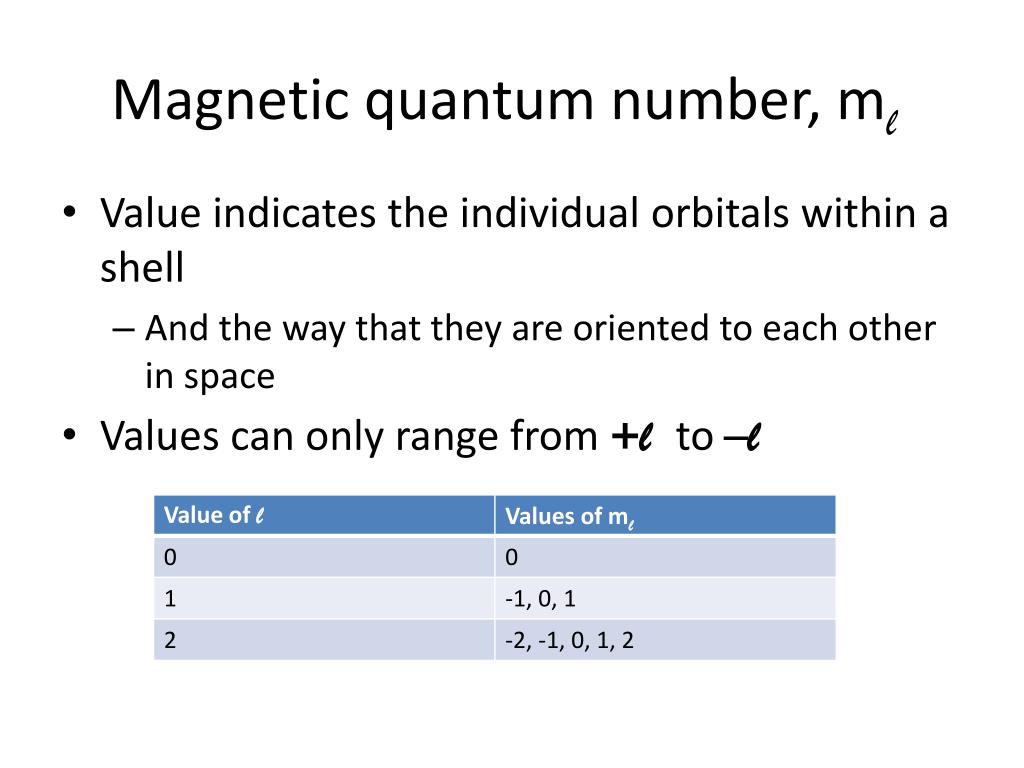
PPT Principle Quantum Numbers PowerPoint Presentation Free Download
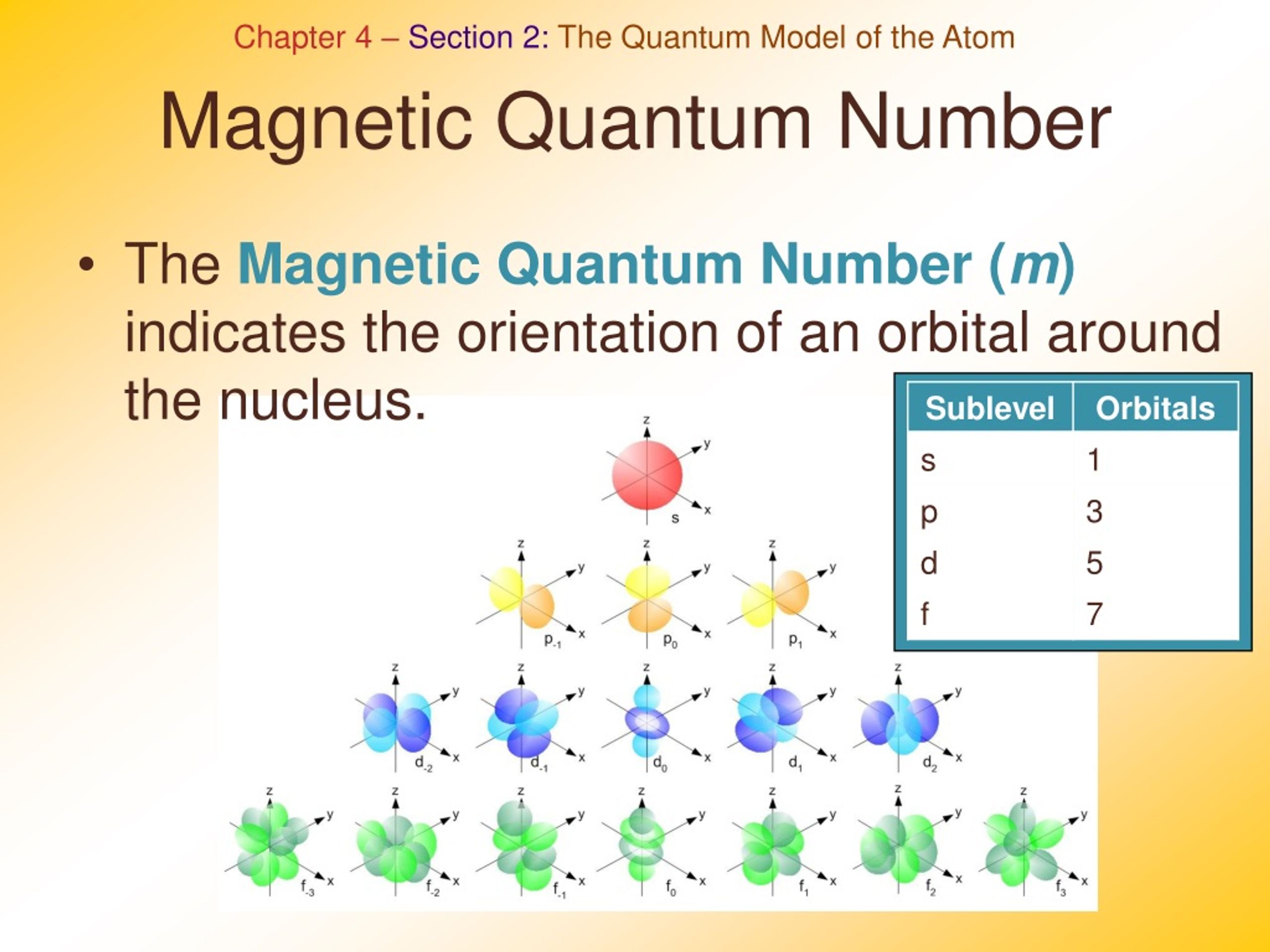
PPT Unit 2 PowerPoint Presentation Free Download ID 298336

Quantum Numbers Principal Angular momentum Magnetic Part 1 YouTube

PPT Quantum Numbers PowerPoint Presentation ID 2135857
Quantum Numbers Types Principal Azimuthal Magnetic And Spin
https://www.toppr.com › ask › question › the-magnetic-quantum-number …
Which of the following statements concerning the quantum numbers are correct a The angular momentum quantum number determines the three dimensional shape of the orbital b The

https://www.toppr.com › ask › question › how-many-electrons-will-have-…
How many maximum number of electrons can be there in an F e 2 ion having principal quantum number n 3 and magnetic moment quantum number I ml 1 View Solution Q 4

https://www.toppr.com › ask › question › if-m-magnetic-quantum-numbe…
The maximum number of electrons with spin value 1 2 in the orbital with azimuthal quantum number value l 2 and magnetic quantum number m 2 is View Solution Q 5

https://www.toppr.com › ask › question
The maximum number of electrons that can have principal quantum number n 4 magnetic quantum number m 1 and spin quantum number m s 1 2 are View Solution Q 5
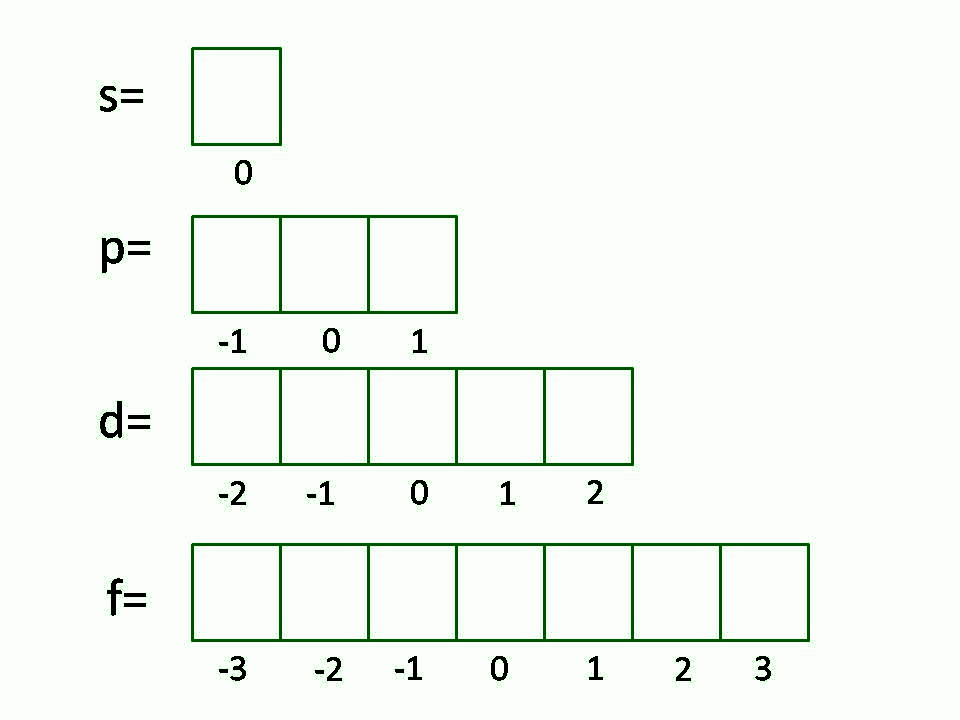
https://www.toppr.com › ask › question › if-highest-magnetic-quantum-n…
What is likely to be principal quantum number for a circular orbit of diameter 20 nm of the hydrogen atom if we assume Bohr orbit be the same as that represented by the principal
[desc-11] [desc-12]
[desc-13]