How To Calculate Principal Quantum Number Web About Transcript An orbital is a region around an atom s nucleus where electrons are likely to be found Different types of orbitals s p d f have different shapes and can hold different numbers of electrons Learn how quantum numbers are used to describe the orbitals and compare Bohr model orbits with the quantum mechanical model of atom
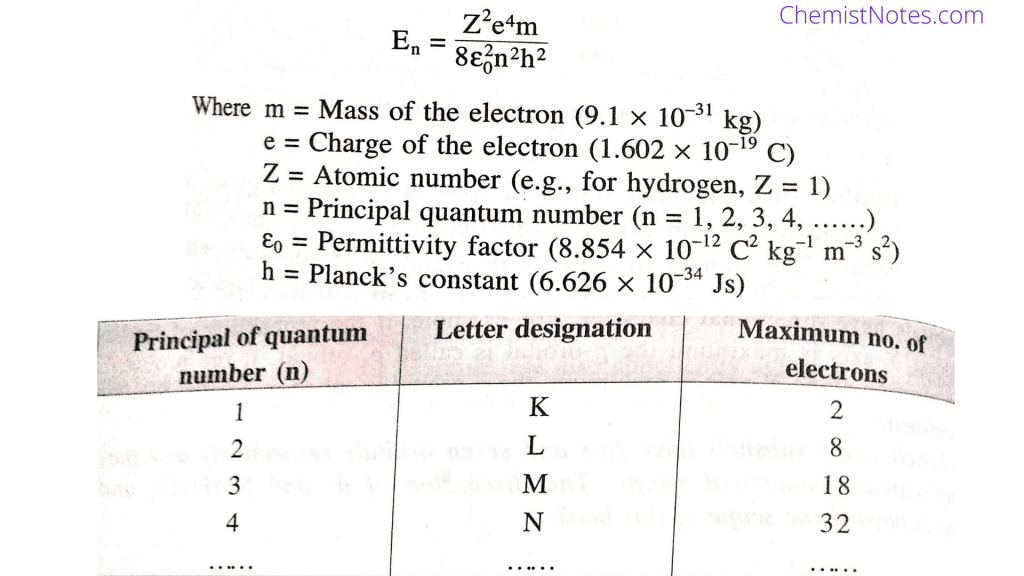
Web E 13 6 eV Z 2 n 2 Where E energy Z atomic number n principal quantum number P r i n c i p a l Q u a n t u m N u m b e r How to Find Principal Quantum Number The principal quantum number of an element can be determined from the periodic table The elements are designated by their period and group Web Principal quantum number n 3 Azimuthal quantum number l 2 Magnetic quantum number ml 2 1 0 1 2 Question Calculate the minimum and maximum number of electrons which have magnetic quantum number m 1 and spin quantum number s 1 2 in chromium Solution Spin can either be 1 2 or 1 2 So it is 2 electrons
How To Calculate Principal Quantum Number
 How To Calculate Principal Quantum Number
How To Calculate Principal Quantum Number
https://image3.slideserve.com/5520888/principal-quantum-number-n.jpg
Web There are eight main shells referring to the principal quantum number n 1 2 3 4 5 6 7 8 that describes atomic orbitals There are four major subshells s p d and f whose names derive from spectroscopic descriptions of
Templates are pre-designed documents or files that can be used for different purposes. They can save effort and time by providing a ready-made format and design for creating various kinds of content. Templates can be used for individual or professional tasks, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
How To Calculate Principal Quantum Number

Homework And Exercises Calculating With Quantum Numbers And Shape Of

Quantum Numbers
Quantum Numbers Types Principal Azimuthal Magnetic And Spin
What Is Quantum Number What Do Quantum Number Determine Tuition Tube
Quantum Numbers Principle Azimuthal Magnetic And Spin

How To Determine The Maximum Number Of Electrons Given A Set Of Quantum

https://byjus.com/chemistry/quantum-numbers
Web The value of the principal quantum number can be any integer with a positive value that is equal to or greater than one The value n 1 denotes the innermost electron shell of an atom which corresponds to the lowest energy state or the ground state of an electron

https://en.wikipedia.org/wiki/Principal_quantum_number
Web The principal quantum number is related to the radial quantum number n r by n n r 1 displaystyle n n r ell 1 where is the azimuthal quantum number and n r is equal to the number of nodes in the radial wavefunction

https://chem.libretexts.org/Bookshelves/Organic...
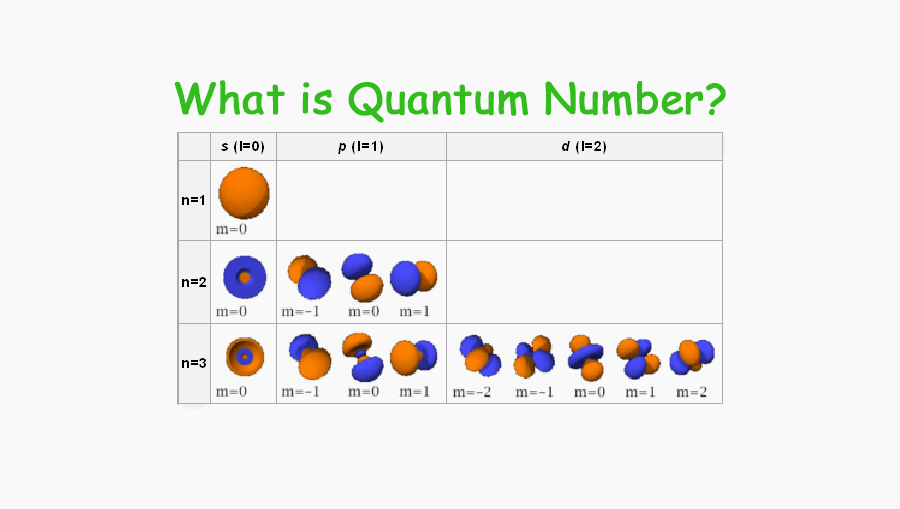
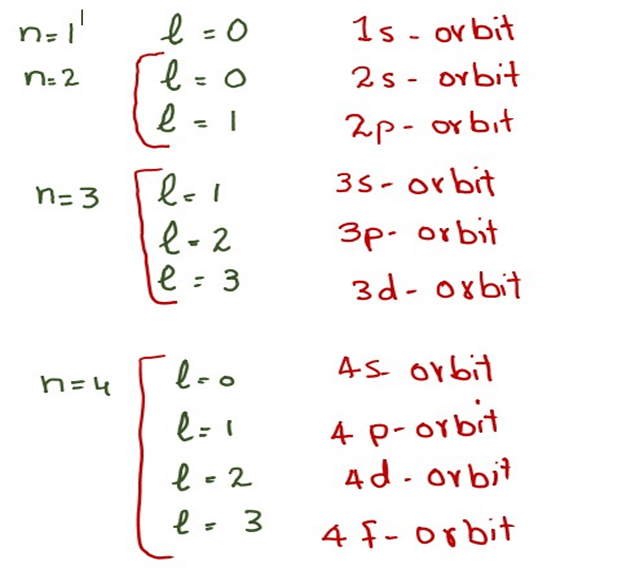
Web 1 PRINCIPAL QUANTUM NUMBER n Represents the main energy level or shell occupied by an electron It is always a positive integer that is n 1 2 3 2 SECONDARY QUANTUM NUMBER l Represents the energy sublevel or

https://chem.libretexts.org/Courses/Oregon...
Web The number before the orbital name such as 2s 3p and so forth stands for the principal quantum number n The letter in the orbital name defines the subshell with a specific angular momentum quantum number l 0 for s orbitals 1 for p orbitals 2 for d orbitals
https://phys.libretexts.org/Bookshelves/College...
Web Feb 20 2022 nbsp 0183 32 We define n n to be the principal quantum number that labels the basic states of a system The lowest energy state has n 1 n 1 the first excited state has n 2 n 2 and so on Thus the allowed values for the principal quantum number are n 1 2 3 30 8 2 30 8 2 n 1 2 3
Web The principal quantum number signified by n is the main energy level occupied by the electron Energy levels are fixed distances from the nucleus of a given atom They are described in whole number increments e g 1 2 3 4 5 6 Web 1 First a mention of the rules for the four quantum numbers n principal quantum number major energy level Values are 1 2 3 azimuthal quantum number energy sublevel Values are 0 to n 1 m magnetic quantum number the orbital in the sublevel Values are 0
Web Nov 21 2023 nbsp 0183 32 The total number of nodes can be calculated by subtracting one from the principal quantum number For example for an atom with n 4 the number of nodes will be 3 The equation for nodes is eq