How To Find Principal Quantum Number From Subshell The number before the orbital name such as 2s 3p and so forth stands for the principal quantum number n The letter in the orbital name defines the subshell with a specific angular momentum quantum number l 0 for s orbitals 1 for p
Free online quantum number calculator for chemistry and physics students Calculate principal angular momentum magnetic and spin quantum numbers with our interactive tools May 22 2018 nbsp 0183 32 Identify the subshell represented by the second or angular quantum number The numbers 0 through 3 represent the quot s quot quot p quot quot d quot and quot f quot subshells respectively For example 1 identifies a quot p quot subshell
How To Find Principal Quantum Number From Subshell
 How To Find Principal Quantum Number From Subshell
How To Find Principal Quantum Number From Subshell
https://i.ytimg.com/vi/z4Zn_AQS3dk/maxresdefault.jpg
The principal quantum number is based on the Bohr model of the atom and it determines which energy level or shell an electron will occupy The principal quantum number can be squared to determine how many orbitals there are
Pre-crafted templates provide a time-saving service for producing a varied series of files and files. These pre-designed formats and designs can be used for numerous personal and professional projects, consisting of resumes, invitations, leaflets, newsletters, reports, presentations, and more, improving the content development procedure.
How To Find Principal Quantum Number From Subshell
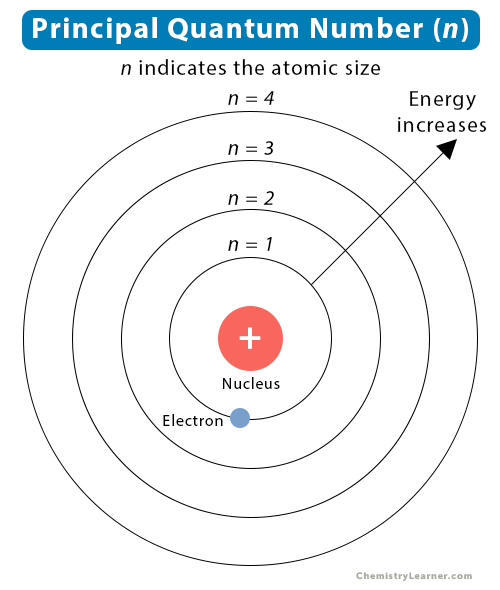
Principal Quantum Number Definition Determination Value
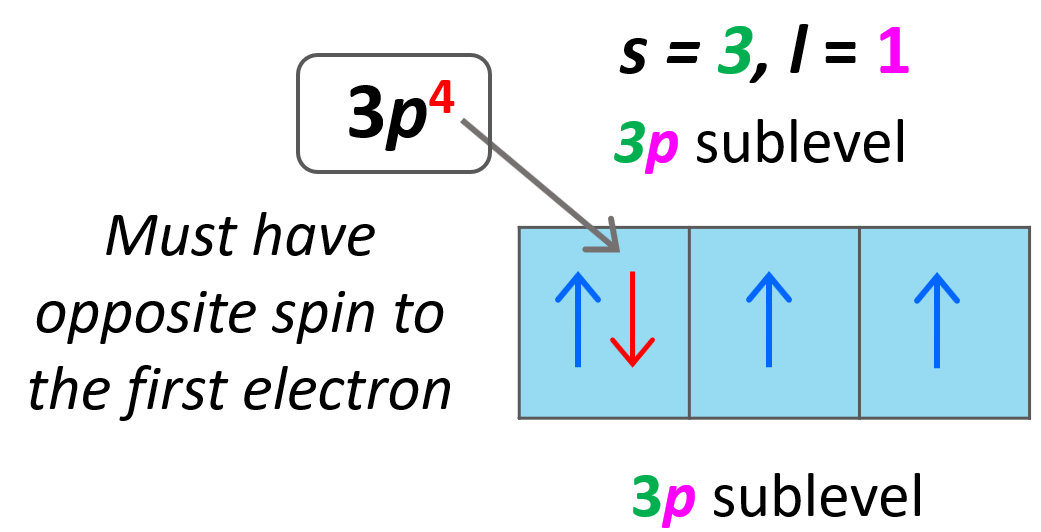
Quantum Numbers Chemistry Steps
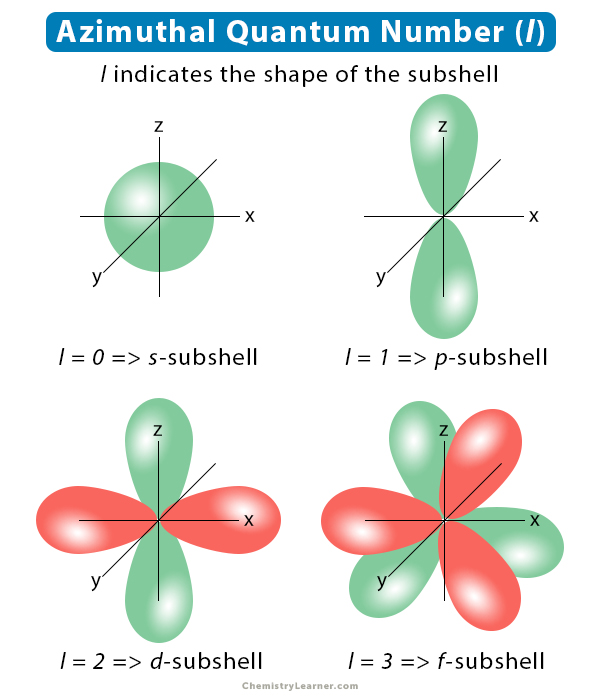
Azimuthal Angular Momentum Quantum Number Definition
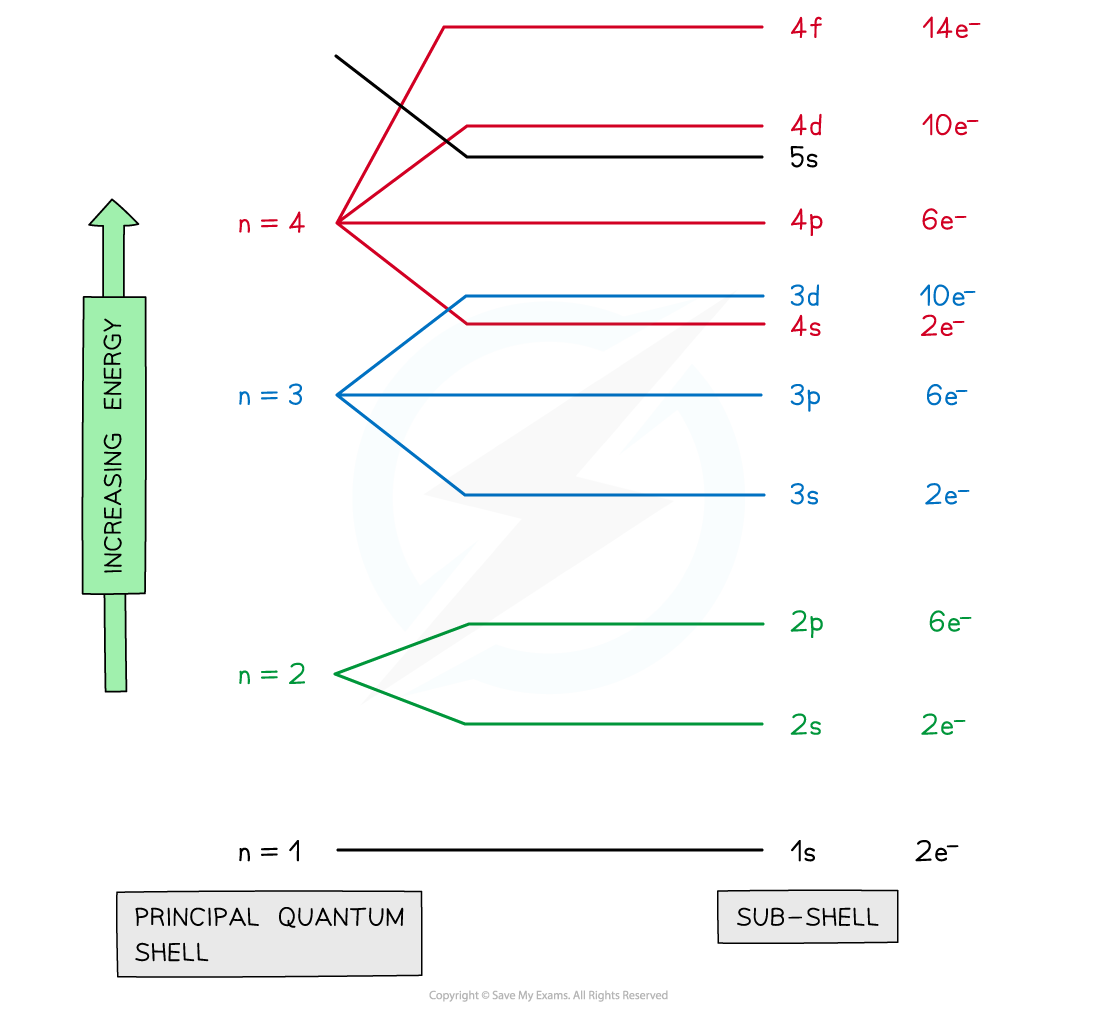
CIE A Level Chemistry 1 1 6 Electronic Structure

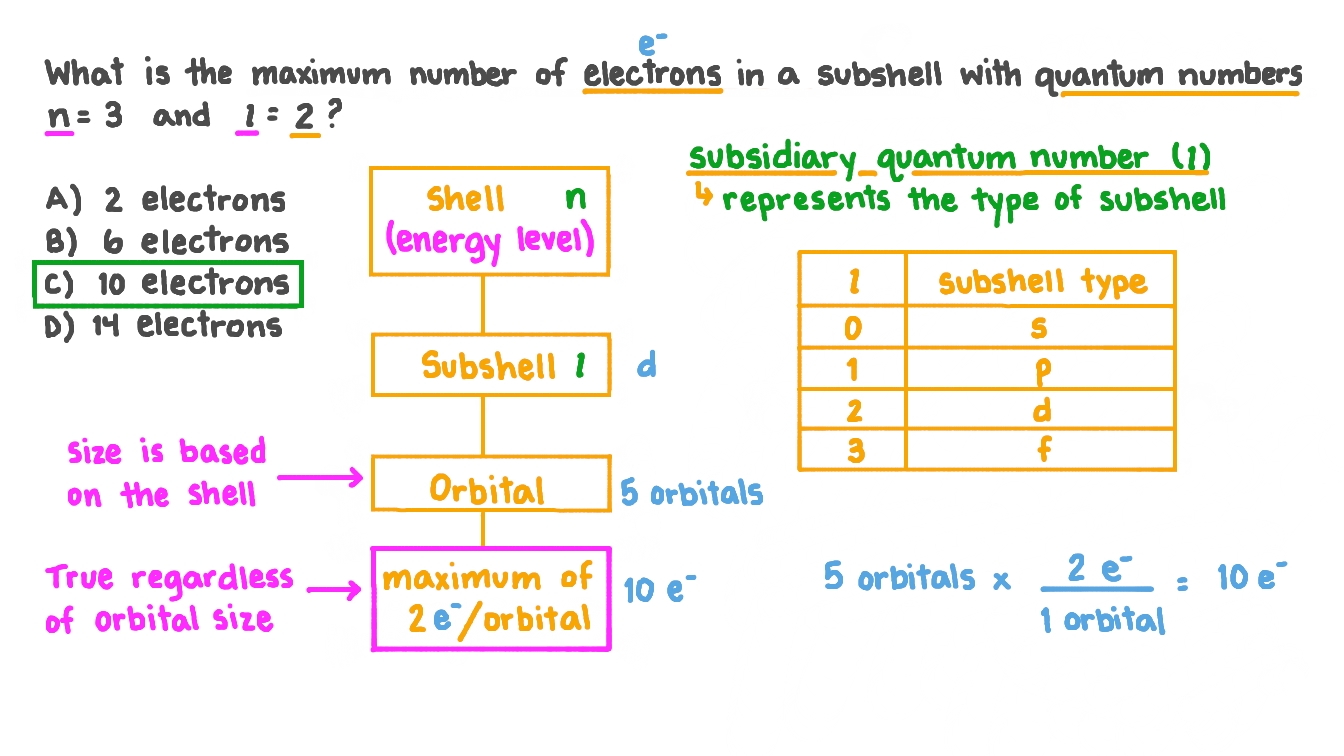
Question Video Determining The Number Of Electrons Using Two Quantum

Quantum Numbers

https://chemed.chem.purdue.edu › ... › bp › …
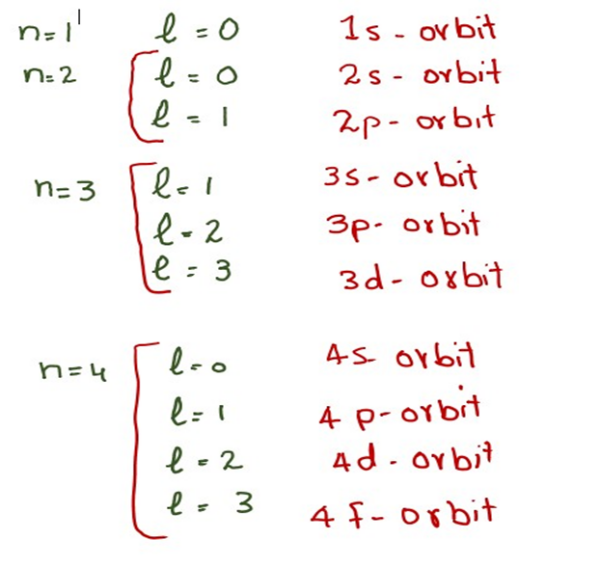
Thus it takes three quantum numbers to define an orbital but four quantum numbers to identify one of the electrons that can occupy the orbital The allowed combinations of n l and m quantum numbers for the first four shells are given

https://revisionscience.com
The number before each subshell specifies which shell it belongs to As an example Lithium has 3 electrons 2 will first fill up the 1st shell in subshell 1s The remaining electron will appear in the second shell in the 2s subshell

https://ecampusontario.pressbooks.pub › ...
Each added electron occupies the subshell of lowest energy available in the order shown in Figure 8 5 1 subject to the limitations imposed by the allowed quantum numbers according to the Pauli exclusion principle
https://www.chemteam.info › Electrons › QuantumNumbers.html
Many problems in this area will ask you to identify an incorrect set of quantum numbers Below the discussion that introduces the rules I have 15 examples with solutions Keep the rules
https://www.chemistrylearner.com › quantu…
How to Find the Principal Quantum Number The principal quantum number of an element s valence electron can be determined from the period in which the element resides in the periodic table For example potassium is in the fourth
In order to find the quantum numbers for electron configuration you need to understand the four different quantum numbers that describe each electron in an atom These quantum numbers Specify the type of quantum number enter the value of n and the calculator will determine the quantum numbers instantly The quantum number calculator allows you to find out the possible
The quantum numbers are parameters that describe the distribution of electrons in the atom and therefore its fundamental nature They are 1 PRINCIPAL QUANTUM NUMBER n