How To Determine Magnetic Spin Quantum Number Web The phrase spin quantum number refers to quantized spin angular momentum The symbol s is used for the spin quantum number and m s is described as the spin magnetic quantum number or as the z component of spin s z Both the total spin and the z component of spin are quantized leading to two quantum numbers spin and spin
Web Jan 30 2023 nbsp 0183 32 The number of subshells or l describes the shape of the orbital It can also be used to determine the number of angular nodes The magnetic quantum number m l describes the energy levels in a subshell and m s refers to the spin on the electron which can either be up or down Web Nov 21 2023 nbsp 0183 32 The spin quantum number is calculated using the inner product of integrals or the Pauli spin matrices For electrons it is possible to calculate whether an electron is spin up or spin down
How To Determine Magnetic Spin Quantum Number
 How To Determine Magnetic Spin Quantum Number
How To Determine Magnetic Spin Quantum Number
https://image3.slideserve.com/6581705/spin-quantum-number-m-s1-l.jpg
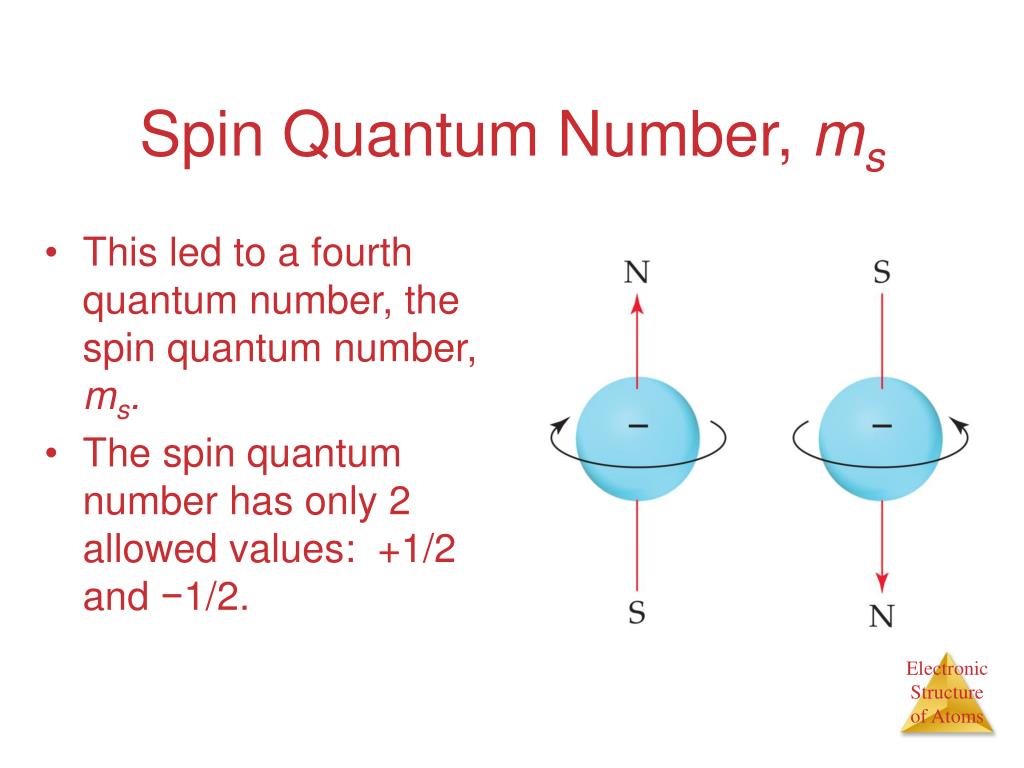
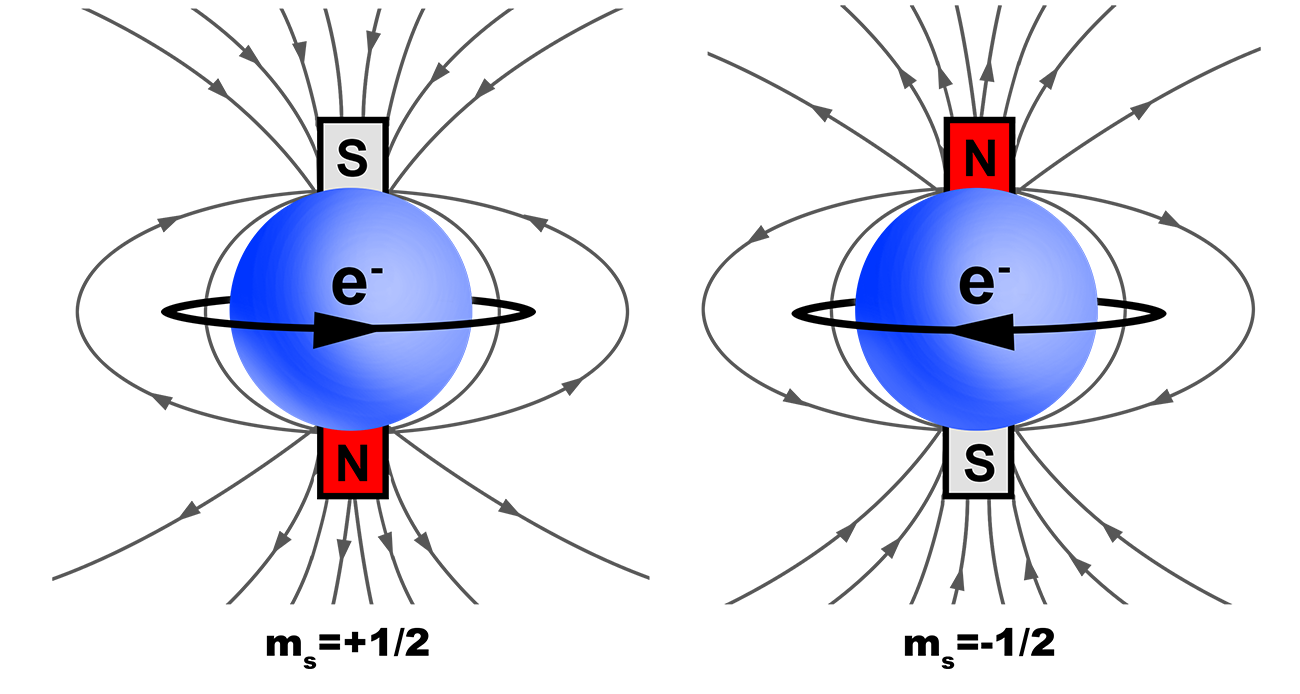
Web Feb 2 2020 nbsp 0183 32 By incorporating electron spin into the electron wave function scientists found that the fourth quantum number also known as the spin quantum number ms could take on two different values they were ms 1 2 and ms 1 2 Notice that unlike n and ml which can only be integers ms can be half integers
Pre-crafted templates use a time-saving option for creating a diverse variety of files and files. These pre-designed formats and designs can be utilized for numerous personal and expert projects, including resumes, invitations, leaflets, newsletters, reports, presentations, and more, enhancing the material development process.
How To Determine Magnetic Spin Quantum Number
Lecture 4 Quantum Lecture 4 Magnetic Quantum Number And Magnetic
Pre Chemistry

The Spin Quantum Number YouTube

UPSC Preparation Quantum Numbers YouTube

188 app

Spin Quantum Number Definition Example Video Lesson Transcript

https://sciencing.com/spin-quantum-number...
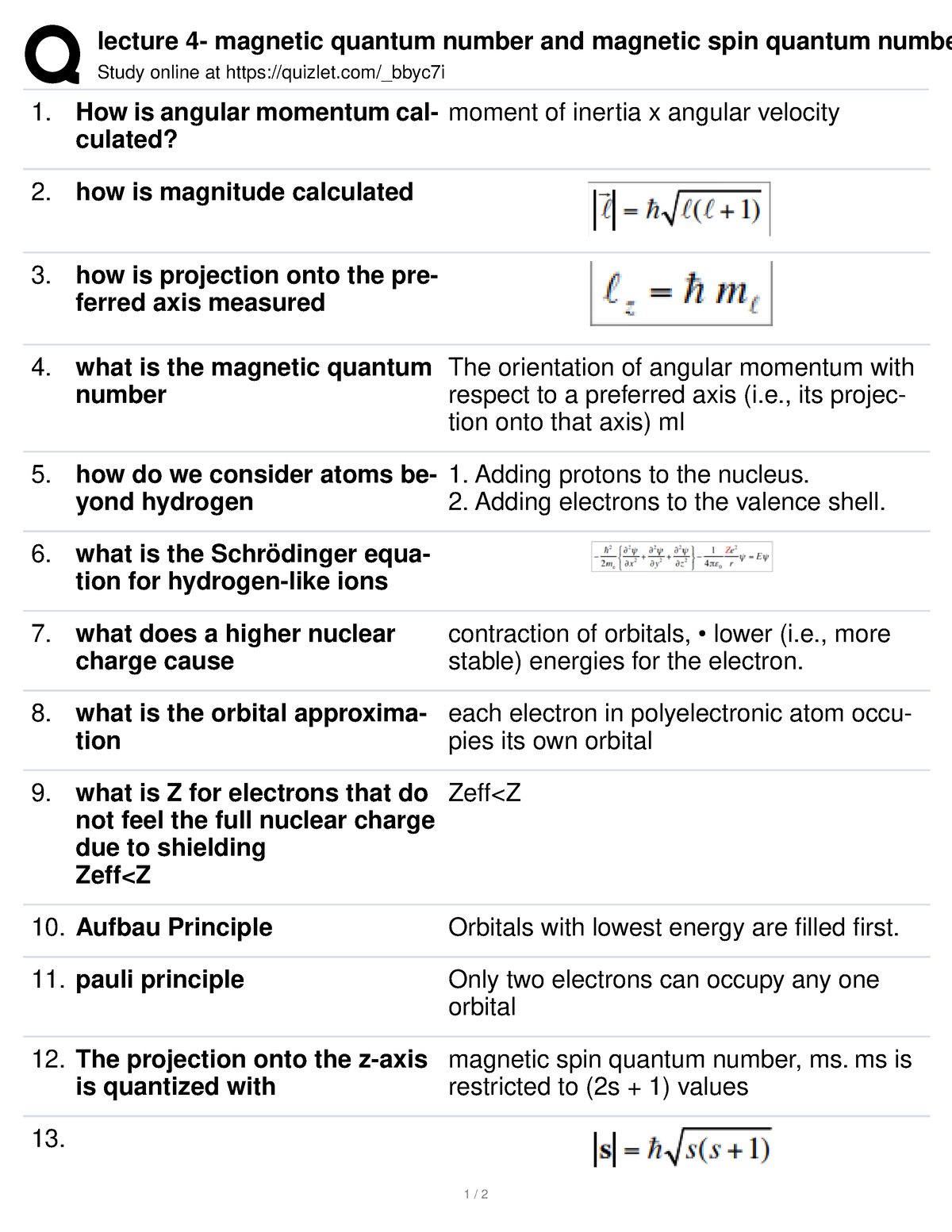
Web Dec 28 2020 nbsp 0183 32 Within an atom each electron is described by four quantum numbers that tell you what state that electron is in and what it s doing These quantum numbers are the principal quantum number n the azimuthal quantum number l the magnetic quantum number m and the spin quantum number s

https://byjus.com/chemistry/spin-quantum-number
Web Determination of Magnetic Nature This quantum number helps to explain the magnetic properties of the substances A spinning electron behaves like a micromagnet with a definite magnetic moment If an orbital contains two electrons then their magnetic moment opposes and cancels each other

https://www.ck12.org/flexi/chemistry/quantum...
Web How can one determine the magnetic spin quantum number Flexi Says The magnetic spin quantum number often represented as quot ms quot can only have two possible values 1 2 or 1 2 This number represents the two possible orientations of a particle s spin quot spin up quot 1 2 or quot spin down quot 1 2

https://chem.libretexts.org/Bookshelves/General...
Web The value ml is the magnetic quantum number The possible ml values follow the equation l 1 2 3 l The ml s values represent the orbitals in a subshell which actually contain electrons Because each orbital ml value can contain 2 electrons we can see how many electrons can be contained in a particular orbital subshell

https://byjus.com/physics/magnetic-quantum-number
Web Spin quantum number The magnetic quantum number is the third on the list between spin and azimuthal quantum number It splits the sub shells such as s p d f into individual orbitals and places the electron in one of them It defines the orientation in space of a given orbital of particular energy n and shape I
Web Feb 16 2024 nbsp 0183 32 Quantum numbers are the set of numbers used to describe the position and energy of an electron in an atom There are four types of quantum numbers principal azimuthal magnetic and spin Quantum numbers represent the values of a quantum system s conserved quantities Let s learn about all the quantum numbers in detail in Web Bo external magnetic field strength g gyromagnetic ratio 1H 26 752 13C 6 7 Note that h is a constant and is sometimes denoted as h 2p NMR Active Nuclei nuclear spin quantum number I atomic mass and atomic number Number of spin states 2I 1 number of possible energy levels Even mass nuclei that have even number of neutron
Web The spin quantum number is denoted by the symbol ms The only possible values of ms are 1 2 and 1 2 When ms 1 2 the electron is in spin up state When ms 1 2 the electron is in spin down state Thus the two electrons occupying the same orbital have opposite spins and are paired 1 6