What Is Cap Corrective Action Plan CAP checklists are living blueprints used by laboratories and inspectors to ensure quality and patient safety
The CAP s Quality Management Tools strengthen your knowledge of key laboratory processes and provides information for effective laboratory management For 75 years the CAP has fostered excellence in laboratories and advanced the practice of pathology and laboratory science As medicine technology and pathology have evolved since
What Is Cap Corrective Action Plan
 What Is Cap Corrective Action Plan
What Is Cap Corrective Action Plan
https://images.sampletemplates.com/wp-content/uploads/2016/04/18072327/corrective-action-form-template.jpeg
CAP Personnel Guidance Document This document illustrates the minimum qualifications for meeting the CLIA and CAP personnel qualifications To assess qualifications identify the
Pre-crafted templates provide a time-saving service for developing a varied range of documents and files. These pre-designed formats and layouts can be used for various personal and expert projects, consisting of resumes, invites, flyers, newsletters, reports, presentations, and more, improving the content production procedure.
What Is Cap Corrective Action Plan
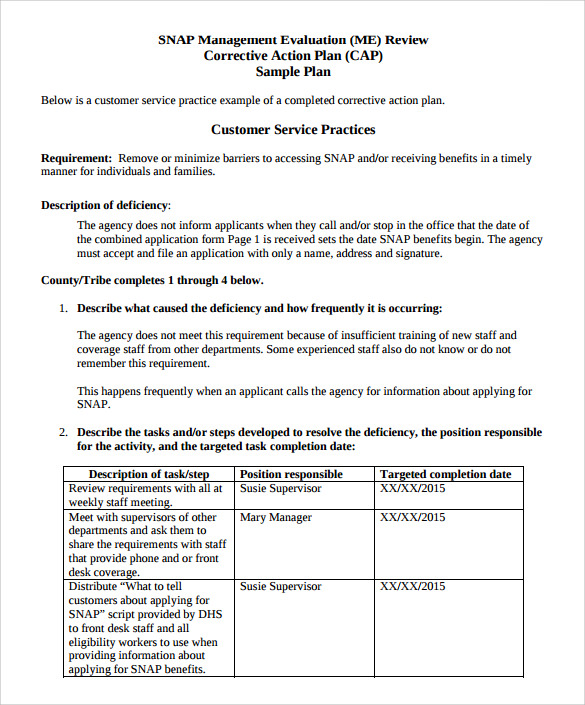
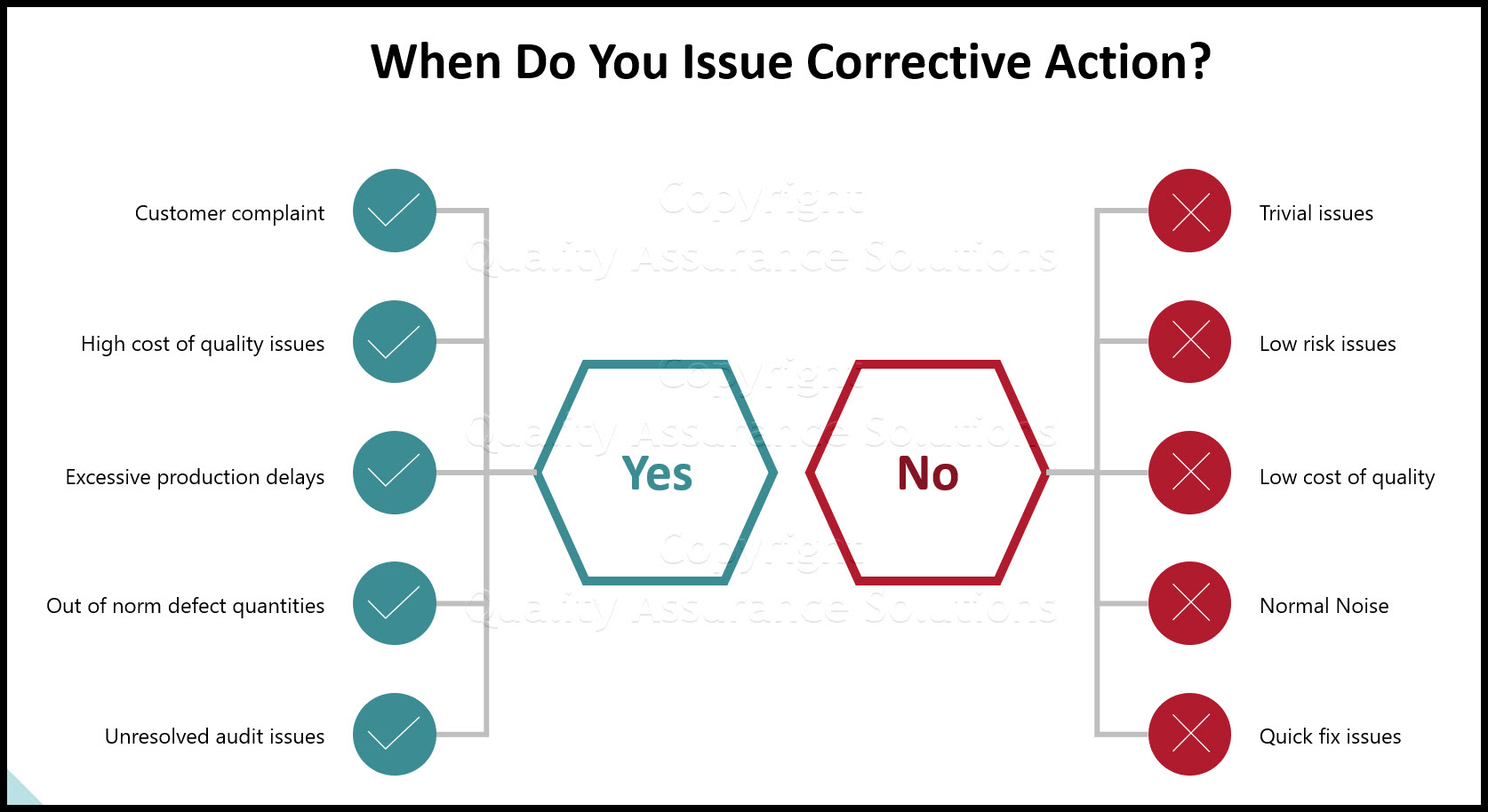
Iso 9001 Corrective Action Examples Gogolasopa

12 Corrective Action Report Examples Pdf Examples Regarding
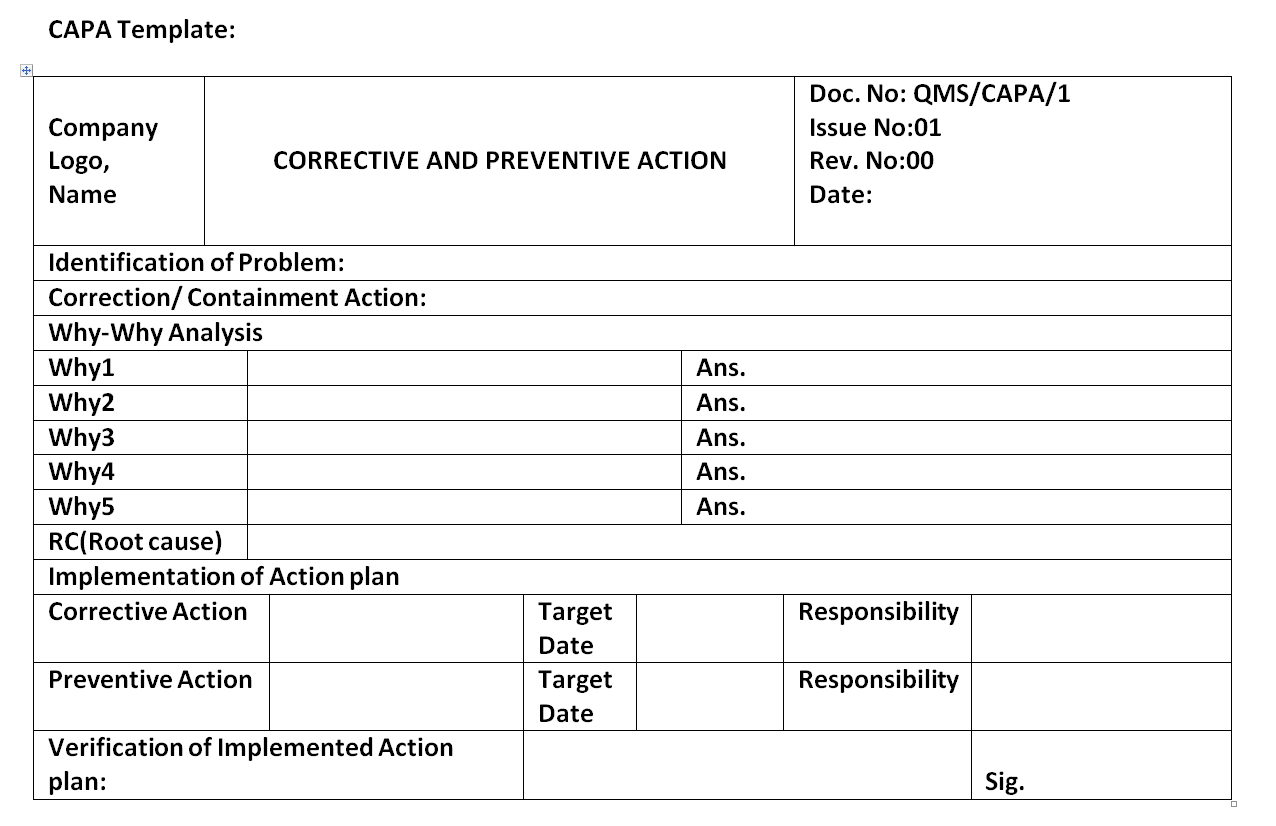
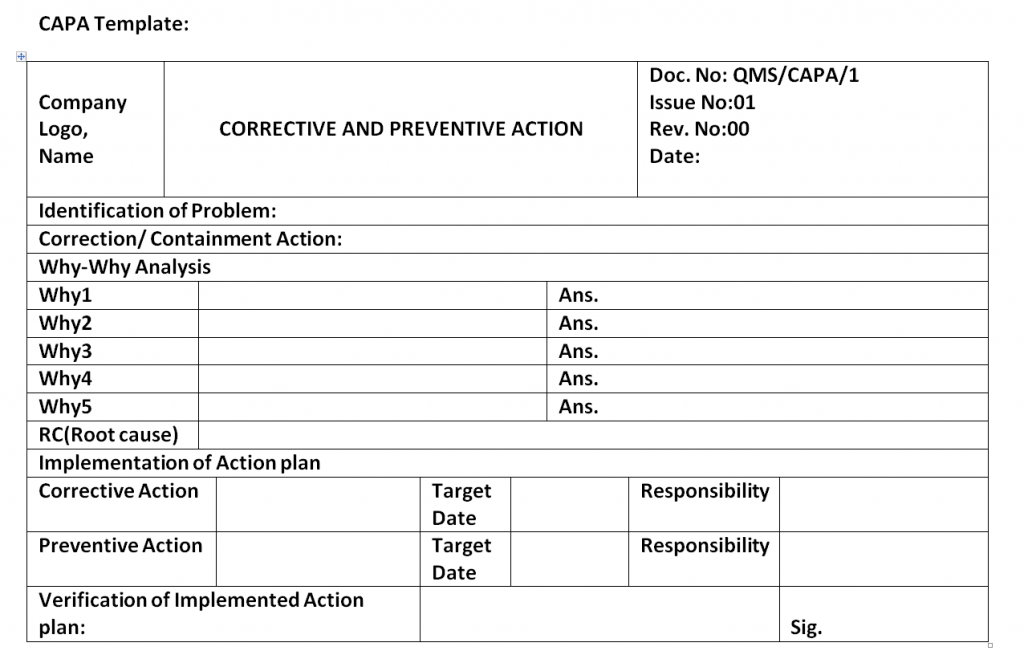
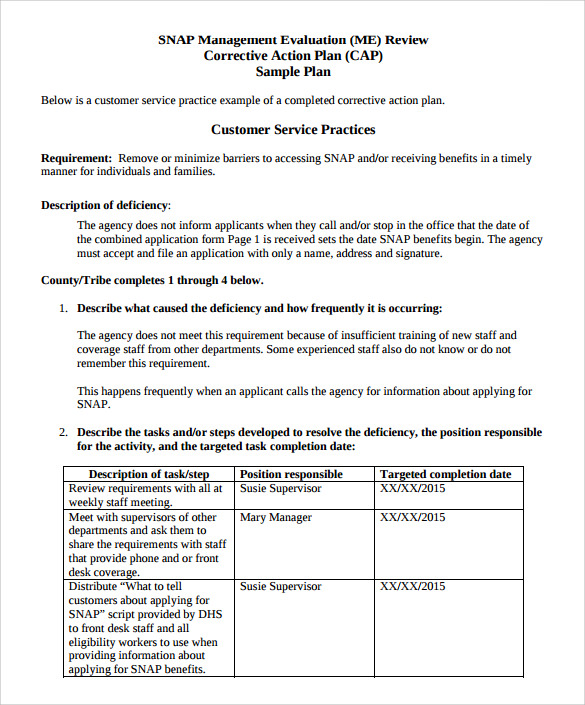
Capa Report Template

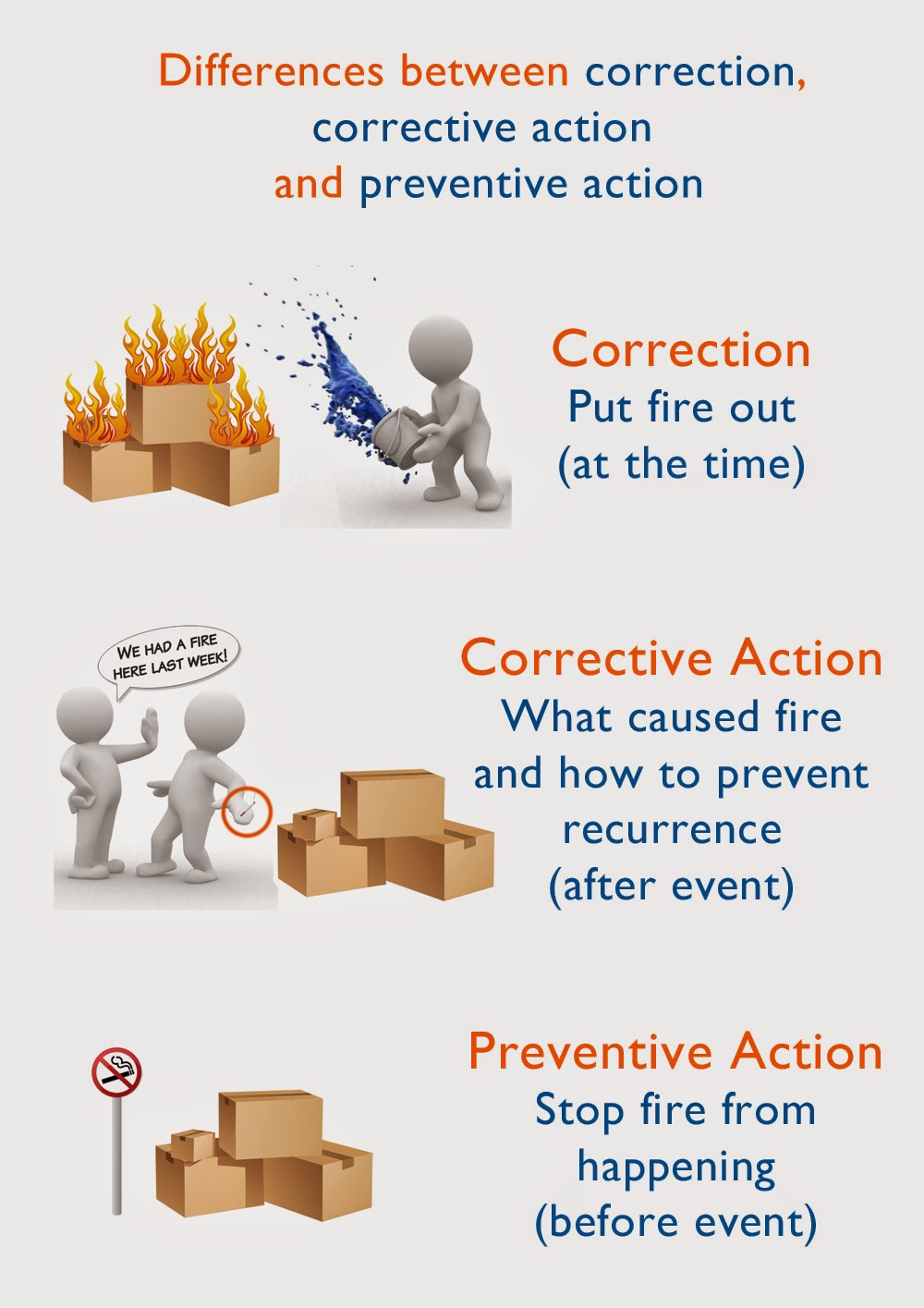
Corrective Action Plan Emmamcintyrephotography
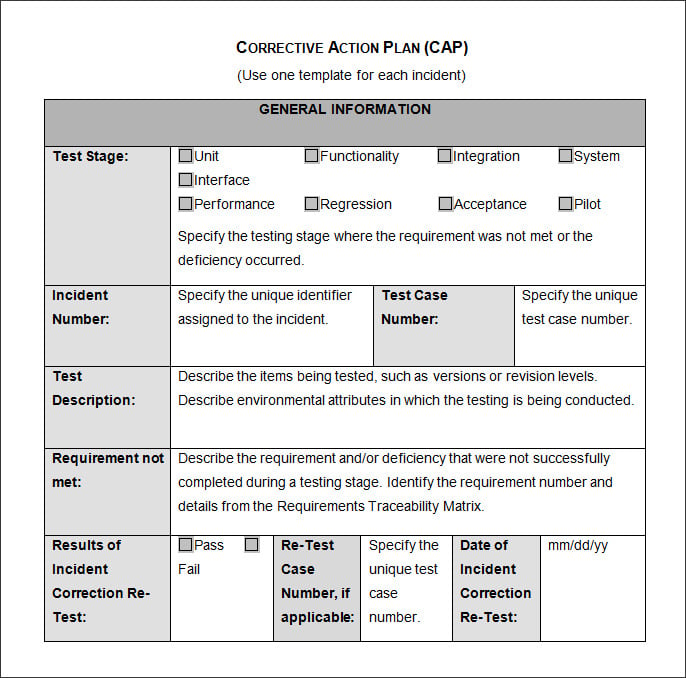
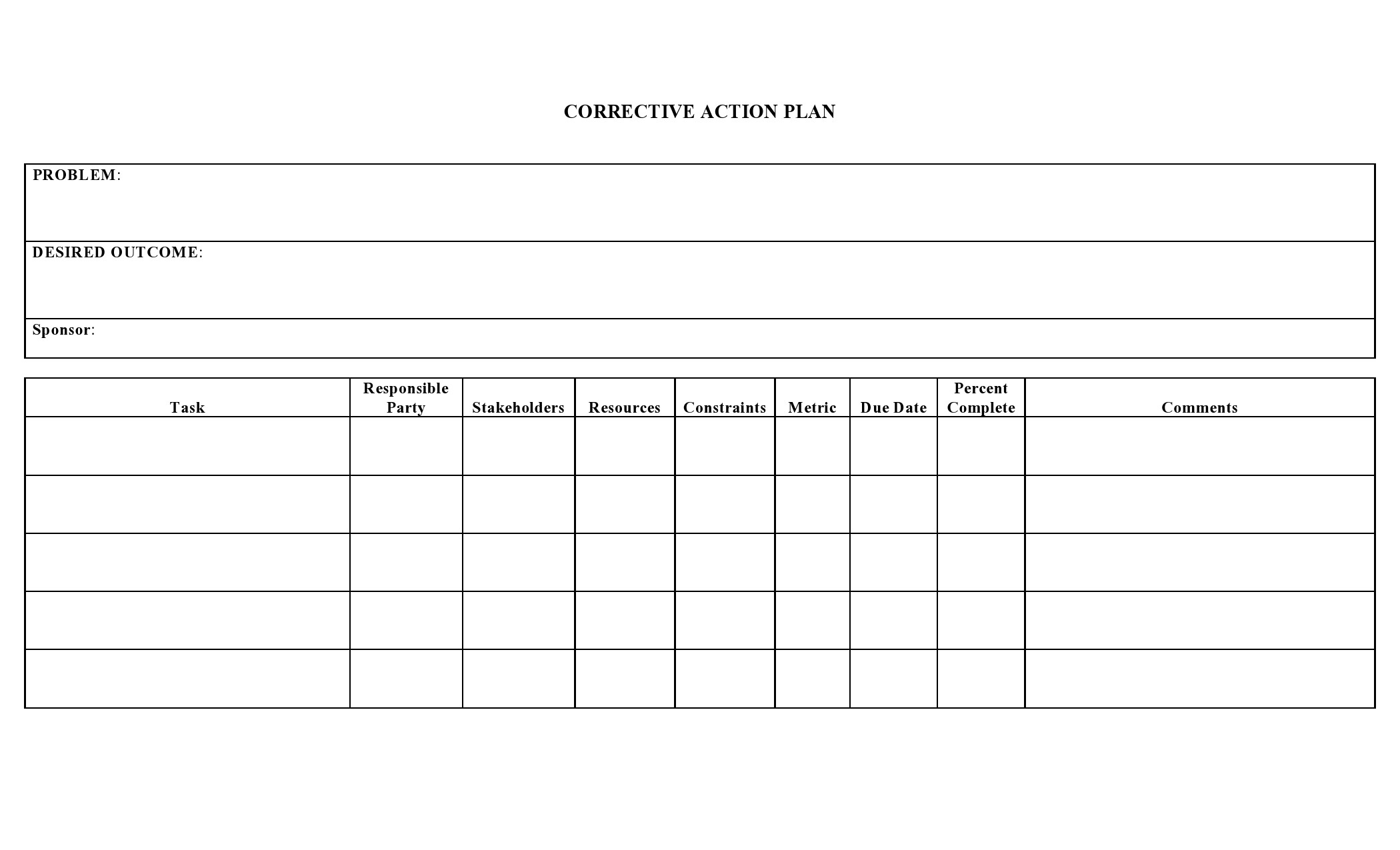
Corrective Action Plan Form
Capa Form Template Free Printable Form Templates And Letter
https://www.cap.org
The CAP hosted a media briefing about how pathologists and clinical laboratories are diagnosing and managing avian influenza bird flu Experts provided insights on how pathologists

https://www.cap.org › contact
Contact the CAP by entering your questions or comments into a brief questionaire Contact Us
https://www.cap.org › laboratory-improvement › accreditation
CAP accredited laboratories around the world demonstrate excellence in patient care and confidence in laboratory practices Learn More
https://www.cap.org › protocols-and-guidelines
The CAP Cancer Reporting and Biomarker Reporting Protocols provide consistent and meaningful information that enable health care professionals to manage and study clinical data

https://documents.cap.org › documents › guide-to-accredit…
As a result CAP Accreditation is globally recognized as the most rigorous choice to achieve and maintain regulatory compliance Our annually updated accreditation checklists provide a road
[desc-11] [desc-12]
[desc-13]