What Do You Mean By Magnetic Quantum Number Feb 8 2025 nbsp 0183 32 Solved Examples on Quantum Numbers Example 1 Write down all the other quantum numbers of an electron for the principal quantum number n 3 Solution Principal
Nov 21 2023 nbsp 0183 32 In this lesson we ll discuss the magnetic quantum number also known as the third quantum number subshells and orbitals and their relationships to one another May 27 2024 nbsp 0183 32 The magnetic quantum number symbolized as m l is an integral value that determines the orientation of an electron s angular momentum in a magnetic field It s derived
What Do You Mean By Magnetic Quantum Number
 What Do You Mean By Magnetic Quantum Number
What Do You Mean By Magnetic Quantum Number
https://i.ytimg.com/vi/KwDZp1-o4lQ/maxresdefault.jpg
The Magnetic Quantum Number often denoted as m l is one of the four quantum numbers that describe the unique quantum state of an electron It designates the orientation of the electron s
Pre-crafted templates provide a time-saving service for developing a varied variety of files and files. These pre-designed formats and designs can be used for numerous individual and professional jobs, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, streamlining the material creation procedure.
What Do You Mean By Magnetic Quantum Number
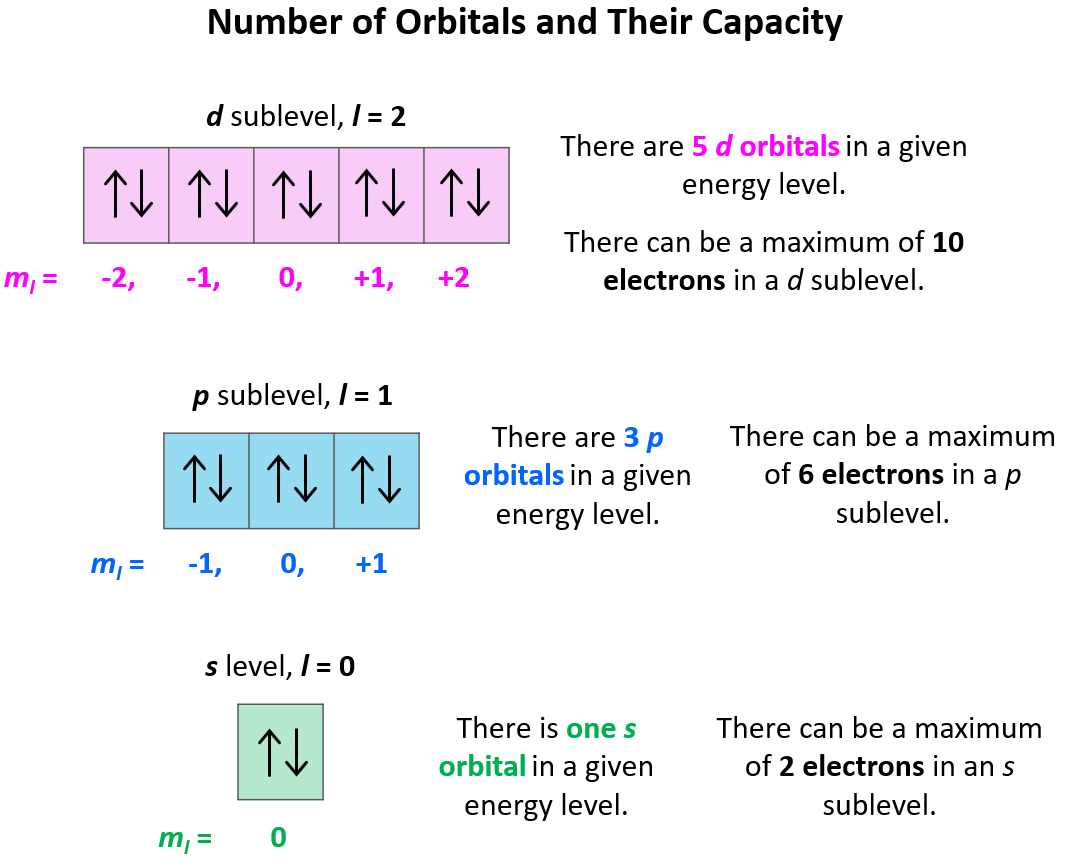
Quantum Numbers Chemistry Steps
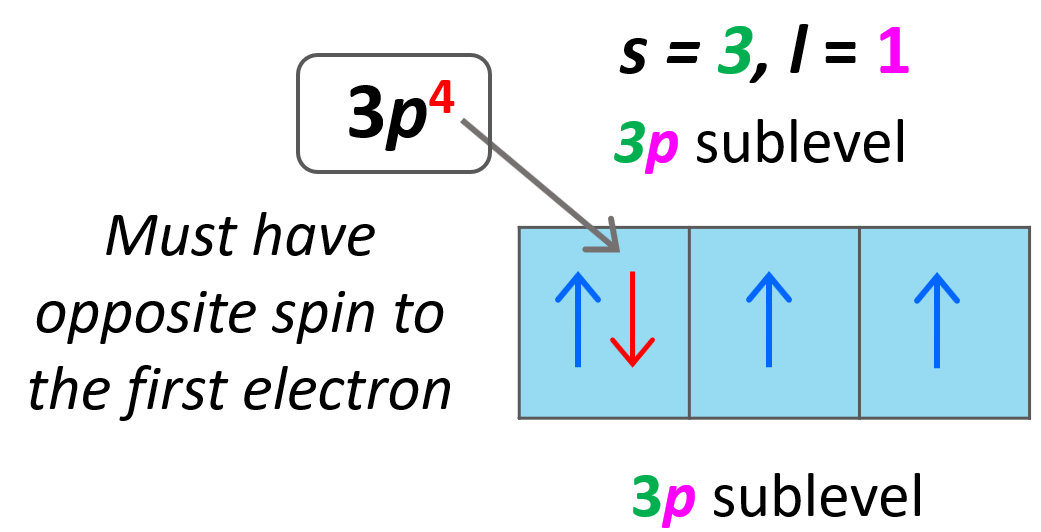
Quantum Numbers Chemistry Steps
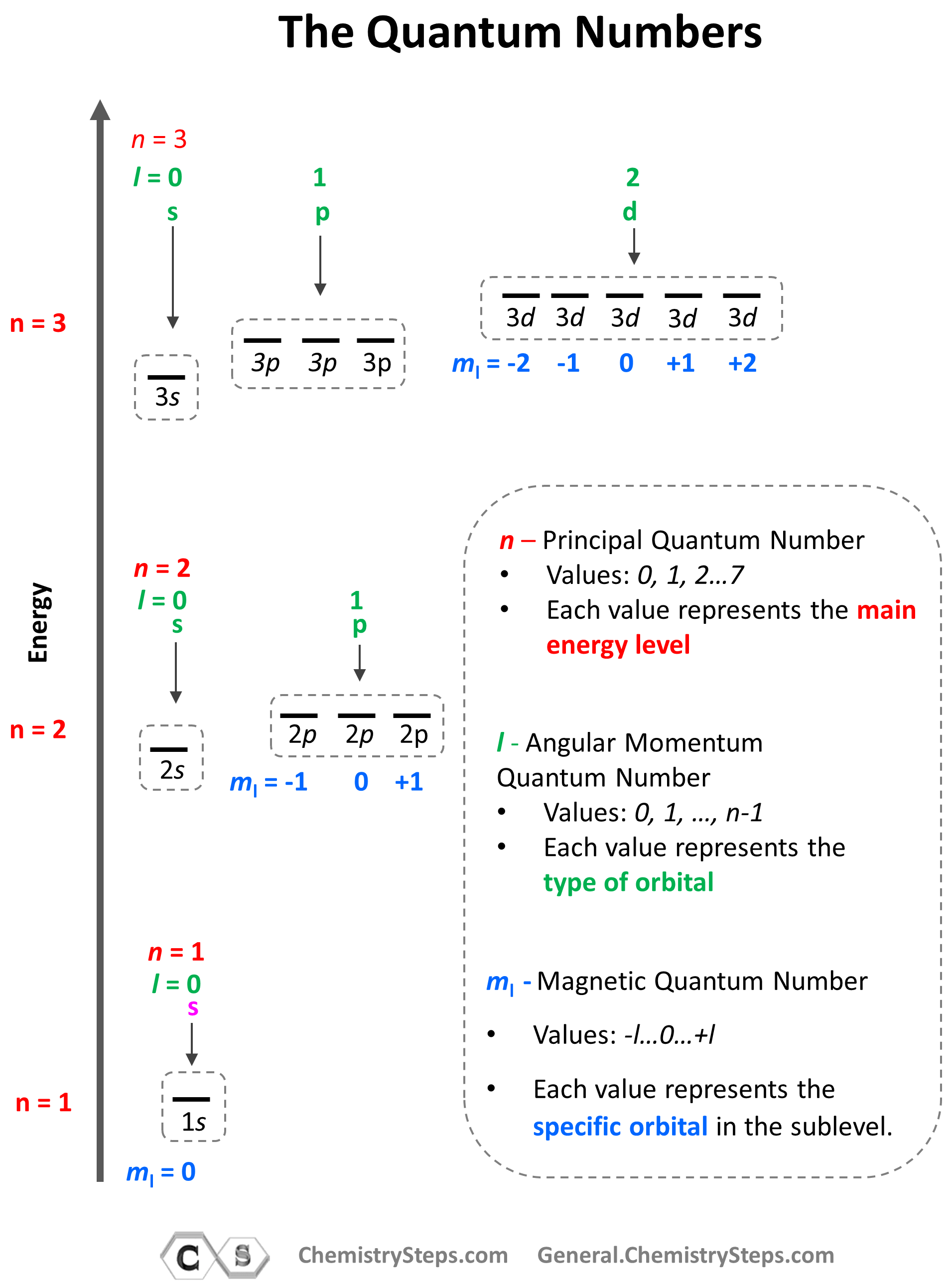
Quantum Numbers Chemistry Steps

Isabelle Miller Let s Gooo Instagram

Spin Quantum Number Definition Significance And Value

Cr 3lixxf On Tt

https://byjus.com › physics › magnetic-quantum-number
The magnetic quantum number is the third on the list between spin and azimuthal quantum number It splits the sub shells such as s p d f into individual orbitals and places the electron

https://byjus.com › chemistry › quantum-numbers
Magnetic Quantum Number The total number of orbitals in a subshell and the orientation of these orbitals are determined by the magnetic quantum number It is denoted by the symbol m l

https://www.chemistrylearner.com › magnetic-quantum-number.html
The magnetic quantum number divides the subshell into orbitals and determines their number Each value of the magnetic quantum number represents a specific orientation of the orbital

https://testbook.com › chemistry › magnetic-quantum-number
Feb 18 2025 nbsp 0183 32 The magnetic quantum number ml m l together with the principal n azimuthal l and spin ms m s quantum numbers is one of four quantum numbers used in atomic

https://www.chemistrylearner.com › quantum-numbers.html
Magnetic Quantum Number m l The magnetic quantum number describes the split in the electron s energy sublevel into two or more levels It is used to project the angular momentum
In atomic physics the magnetic quantum number is the third of a set of quantum numbers the principal quantum number the azimuthal quantum number the magnetic quantum number May 21 2024 nbsp 0183 32 What Is the Magnetic Quantum Number The magnetic quantum number is represented by the letter m or ml This number is used to explain how an atom s electron is
Jan 15 2025 nbsp 0183 32 The magnetic quantum number denoted by m l l l is one of the four quantum numbers that describe the unique quantum state of an electron in an atom It specifies the