Magnetic Spin Quantum Number Example Spin Quantum Number Spin Quantum Number represents the direction of the spin of the electrons This can either be in the direction of clockwise or even anti clockwise Spin Quantum Number is denoted by the symbol s It can have about only two values i e 1 2 or 1 2 Solved Examples for You Question An electron is in one of the 3d orbitals
The phrase spin quantum number refers to quantized spin angular momentum The symbol s is used for the spin quantum number and m s is described as the spin magnetic quantum number 3 or as the z component of spin s z 4 Both the total spin and the z component of spin are quantized leading to two quantum numbers spin and spin magnet quantum Jul 1 2024 nbsp 0183 32 The spin quantum number indicates the number of electrons that exist in an orbital For each magnetic quantum number there are only two values are obtained from the spin quantum number Those obtained values are 1 2 and 1 2
Magnetic Spin Quantum Number Example
 Magnetic Spin Quantum Number Example
Magnetic Spin Quantum Number Example
https://i.ytimg.com/vi/Yxpn8VCBPsQ/maxresdefault.jpg
The Magnetic Quantum Number determines the orientation of an electron s orbital in a magnetic field while the Spin Quantum Number characterizes the intrinsic angular momentum or spin of a particle itself
Templates are pre-designed files or files that can be used for different purposes. They can save effort and time by providing a ready-made format and design for creating different type of content. Templates can be utilized for individual or professional projects, such as resumes, invitations, flyers, newsletters, reports, discussions, and more.
Magnetic Spin Quantum Number Example

Understanding Magnetic Quantum Number In Quantum Mechanics YouTube

Quantum Numbers Magnetic Quantum Number Ml Chem161 7 6 YouTube

Calculation Of Nuclear Spin Nuclear Shell Model YouTube

Azimuthal Quantum Number Teaching Chemistry Science Facts Quantum
Atomic Structure Archives Chemistry Notes
Quantum Numbers Principle Azimuthal Magnetic And Spin

https://scienly.com › quantum-numbers
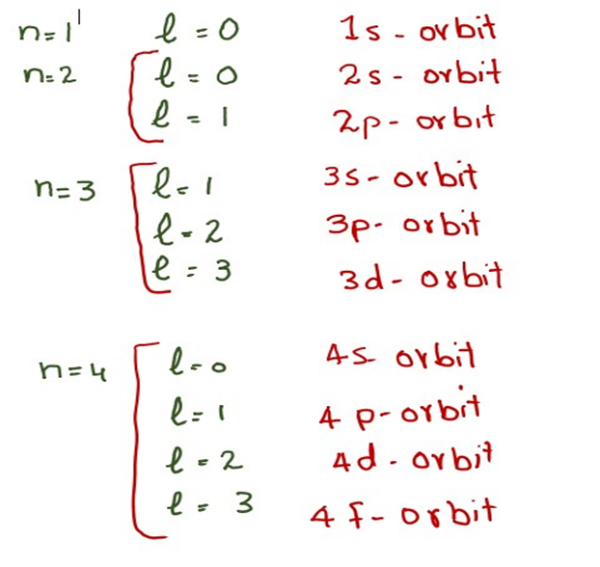
Feb 8 2025 nbsp 0183 32 Solved Examples on Quantum Numbers Example 1 Write down all the other quantum numbers of an electron for the principal quantum number n 3 Solution Principal quantum number n 3 given The possible values of azimuthal quantum number l 0 to n 1 0 1 2 The possible values of magnetic quantum number m l to l including 0

https://byjus.com › chemistry › quantum-numbers
Magnetic Quantum Number The total number of orbitals in a subshell and the orientation of these orbitals are determined by the magnetic quantum number It is denoted by the symbol m l This number yields the projection of the angular momentum corresponding to the orbital along a

https://eduinput.com › examples-of-quantum-numbers
Oct 11 2023 nbsp 0183 32 Magnetic Quantum Number m and Orbital Orientation The magnetic quantum number m specifies the orientation of an orbital in space For a given l value there are 2l 1 possible values of m For example in a p orbital l 1 m can be 1 0 or 1 The spin quantum number m describes the intrinsic spin of an electron

https://www.geeksforgeeks.org › quantum-numbers
Feb 16 2024 nbsp 0183 32 The magnetic quantum number is determined by the azimuthal or orbital angular momentum quantum number For a given value of l the value of m l falls between l to l As a result it is indirectly dependent on the value of n For example if n 4 and l 3 in an atom the magnetic quantum number can be 3 2 1 0 1 2 and 3

https://www.chemistrylearner.com › quantum-numbers.html
The magnetic quantum number describes the split in the electron s energy sublevel into two or more levels It is used to project the angular momentum along a specific axis The number of splits is the total number of values that m l can take
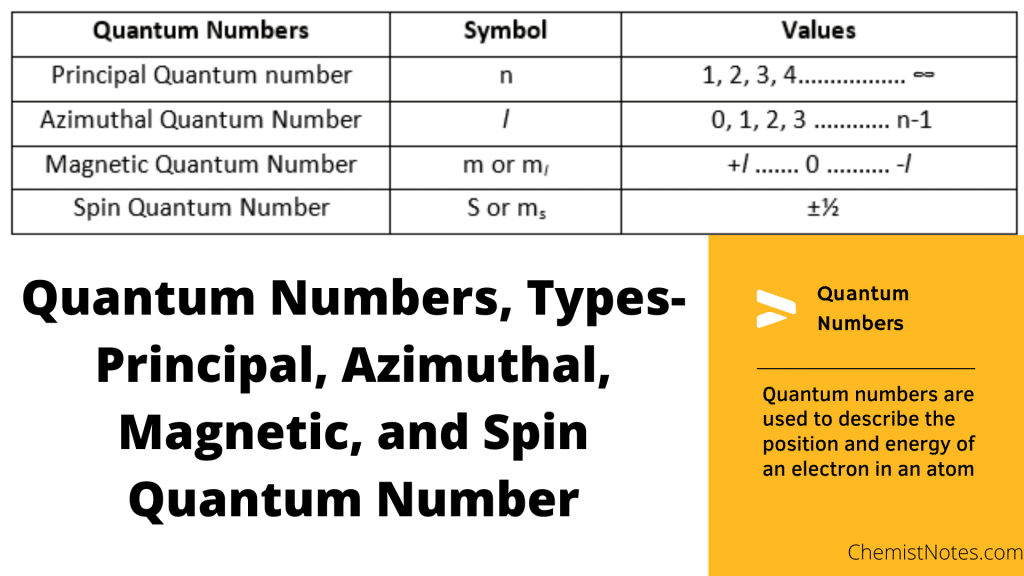
Magnetic quantum number The third quantum number m defines the orientation of an electron wave or orbital with respect to a given direction in presence of a strong electric or magnetic field It does not affect the shape of the orbital or the energy of an electron but uses to specify the sub level of an atom How to find the magnetic quantum Dec 28 2020 nbsp 0183 32 Within an atom each electron is described by four quantum numbers which tell you what state that electron is in and what it s doing These quantum numbers are the principal quantum number n the azimuthal quantum number l the magnetic quantum number m and the spin quantum number s
Jun 29 2023 nbsp 0183 32 There are four main quantum numbers primary quantum number n secondary quantum number l magnetic quantum number m and spin quantum number s The principal quantum number n represents the energy levels of the