How To Find The Spin Quantum Number Of An Element WEB m s spin quantum number electron in orbital Values are 1 2 or 1 2 2 The problem with a is in the m s value The error is that m s 1 The m s value can only be 1 2 or 1 2 3 The problem with b lies in the relationship between n and Since n 1 in b the value for MUST be 0
WEB The spin quantum number is denoted by the symbol ms The only possible values of ms are 1 2 and 1 2 When ms 1 2 the electron is in spin up state When ms 1 2 the electron is in spin down state WEB Nov 21 2023 nbsp 0183 32 The spin quantum number tells us the orientation of an electron within an orbital and has two possible values ms 1 2 for spin up and ms 1 2 for spin down A maximum number of two electrons
How To Find The Spin Quantum Number Of An Element
 How To Find The Spin Quantum Number Of An Element
How To Find The Spin Quantum Number Of An Element
https://media.nagwa.com/624164217907/en/thumbnail_l.jpeg
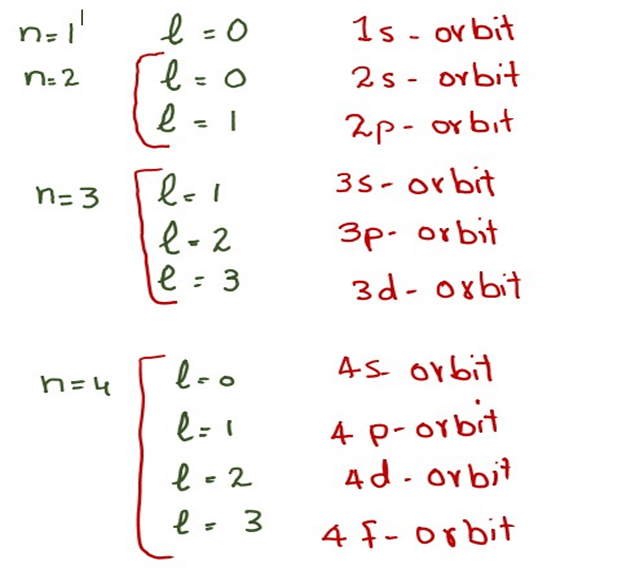
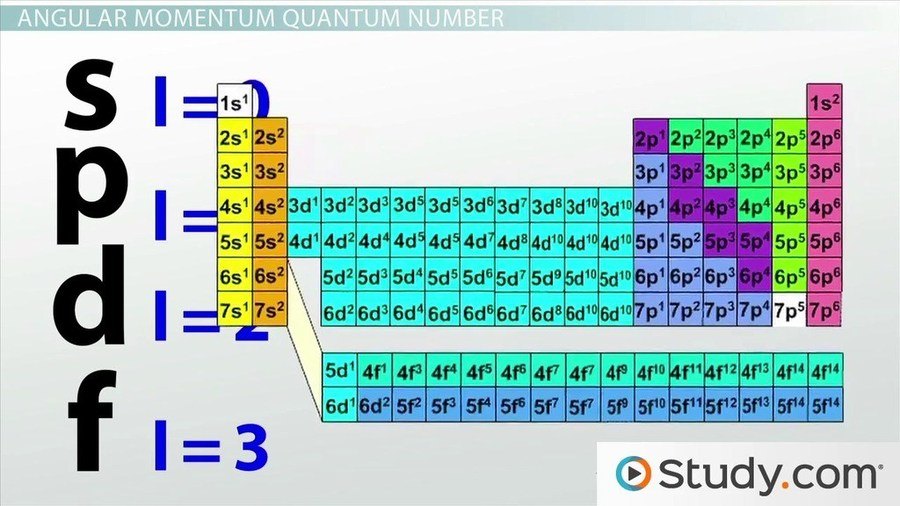
WEB Step 1 Find the element on the periodic table Step 2 Determine n and l by identifying the period and the block that the element is located in Step 3 Determine ml by labeling the block
Templates are pre-designed files or files that can be utilized for different functions. They can save time and effort by offering a ready-made format and design for producing different kinds of material. Templates can be used for individual or professional tasks, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
How To Find The Spin Quantum Number Of An Element

If Spin Quantum Number Ms Can Have Values 12 0 And 12 Then Total

Spin Quantum Numbers

Spin Quantum Numbers ms Deepstash
Quantum Numbers Principle Azimuthal Magnetic And Spin

Spin Quantum Number Definition Significance And Value
Quantum Numbers Chemistry Quizizz

https://byjus.com/chemistry/spin-quantum-number
WEB The spin quantum number is the fourth quantum number which describes the orientation of the electron spin rotation in space To calculate it draw the electronic structure distribute the electron according to Hund s rule then the up arrow shows 189 and arrow down shows 189 Example 8 O 16 Number of electrons is 8

https://sciencing.com/spin-quantum-number...
WEB Dec 28 2020 nbsp 0183 32 In general the spin quantum number is denoted with an s For all electrons however s 189 An associated number m s gives the possible orientations of s in the same way m l gave the possible orientations of l The possible values of m s are integer increments between s and s

https://en.wikipedia.org/wiki/Spin_quantum_number
WEB In physics and chemistry the spin quantum number is a quantum number designated s that describes the intrinsic angular momentum or spin angular momentum or simply spin of an electron or other particle It has the same value for all particles of the same type such as s 1 2 for all electrons

https://chem.libretexts.org/Bookshelves/Physical...
WEB Jan 30 2023 nbsp 0183 32 The fourth quantum number is independent of the first three allowing the first three quantum numbers of two electrons to be the same Since the spin can be 1 2 or 1 2 there are two combinations n 2 l 1 m l 0 m s 1 2

https://chem.libretexts.org/Bookshelves...
WEB Spin Quantum Number left m s right The spin quantum number describes the spin for a given electron An electron can have one of two associated spins left frac 1 2 right spin or left frac 1 2 right spin An electron cannot have zero spin
WEB Jul 3 2017 nbsp 0183 32 m s the magnetic spin quantum number relates the spin of the electron per orbital For instance let s take a look at carbon s electron configuration C He 2s 2 2p 2 It would contain 2 core electrons 4 valence electrons 2 unpaired electrons and three orbitals n 1 2 l 0 1 m l 1 1 WEB In atomic physics the spin quantum number is a quantum number that parametrizes the intrinsic angular momentum or spin angular momentum or simply spin of a given particle The spin quantum number is the fourth of a set of quantum numbers the principal quantum number the azimuthal quantum number the magnetic quantum
WEB 24K 1 4M views 9 years ago This video shows you how to identify or determine the 4 quantum numbers n l ml and ms from an element or valence electron This video provides 3 example