How To Find Magnetic Quantum Number Class 11 Welcome studentsin this video i tell you magnetic quantum number in detail first of all tell you what does magnetic quantum number tells about and then tell
The Magnetic Quantum Number m l The magnetic orbital quantum number m l indicates the orbital s spatial orientation in relation to a conventional set of coordinate axes Integer values between l and l including zero make up the magnetic quantum number m l Here you can find the L30 Magnetic amp spin quantum number Structure of Atom Class 11 defined amp explained in the simplest way possible
How To Find Magnetic Quantum Number Class 11
 How To Find Magnetic Quantum Number Class 11
How To Find Magnetic Quantum Number Class 11
https://i.ytimg.com/vi/KwDZp1-o4lQ/maxresdefault.jpg
May 2 2024 nbsp 0183 32 Magnetic Quantum Number Discussion Due to the angular motion of electrons around the nucleus a magnetic field is produced which interacts with the external magnetic field As a result subshells of definite energy split into three dimensional spatial regions called orbitals Magnetic quantum number MI signifies the orientation of the
Templates are pre-designed documents or files that can be utilized for numerous functions. They can save time and effort by offering a ready-made format and layout for developing various sort of material. Templates can be utilized for personal or expert projects, such as resumes, invites, leaflets, newsletters, reports, presentations, and more.
How To Find Magnetic Quantum Number Class 11
CHEMISTRY MATRICULATION QUANTUM NUMBERS

Spin Quantum Number Definition Significance And Value

Spin Quantum Number Definition Significance And Value

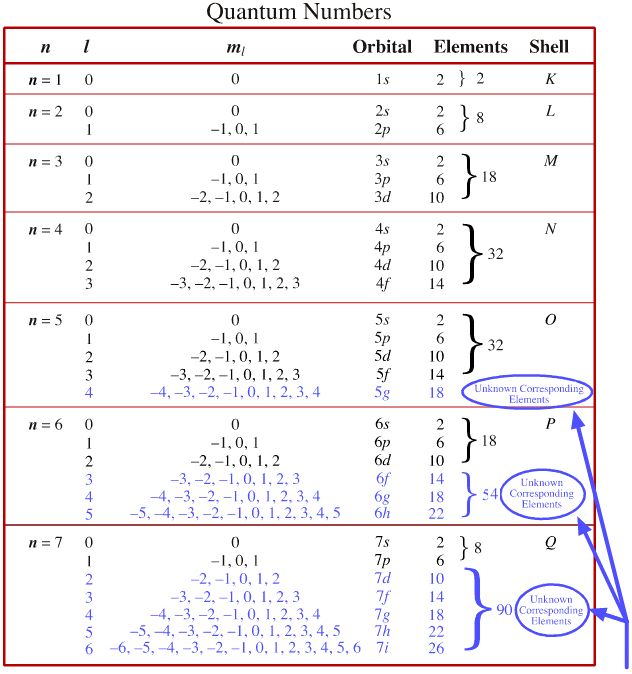
Quantum Numbers

Magnetic Quantum Number Definition Schrodinger Equation

Magnetic Quantum Number Wiki Everipedia

https://byjus.com › chemistry › quantum-numbers
Magnetic Quantum Number The total number of orbitals in a subshell and the orientation of these orbitals are determined by the magnetic quantum number It is denoted by the symbol m l This number yields the projection of the angular momentum corresponding to the orbital along a
:max_bytes(150000):strip_icc()/eyebrow-shape-GettyImages-1194724839-20e5c0d31e924288ac4e6fd442c90598.jpg?w=186)
https://classnotes.org.in › ... › quantum-numbers
Jul 3 2023 nbsp 0183 32 The magnetic quantum number determines the number of preferred orientation of the electron present in a sub shell The magnetic quantum number is denoted by the letter m or m l and for a given value of l it can have all the values ranging from l to l including zero

https://www.toppr.com › ... › quantum-numbers
Magnetic Quantum Number Magnetic Quantum Number denoted by the symbol m is what represents the orientation of atomic orbital in space The value of the Magnetic Quantum Number m depends on the value of l Magnetic Quantum Number can have a total number of 2l 1

https://www.youtube.com › watch
Nov 9 2019 nbsp 0183 32 Magnetic Quantum number animation explains with the help of definition of spin magnetic quantum number Also how to determine magnetic quantum number tips and tricks for

https://www.vedantu.com › question-answer › determine...
The ml represents the Magnetic quantum number and tells the number of orbitals and it can be calculated with the Azimuthal quantum number For any value of l we can calculate the ml as l to 1 We know there are four sub shells i e s p d and f
Quantum numbers for addressing an electronQuantum numbers define the position and energy of an electron in an atomImproving on the Bohr Model Sommerfield t May 14 2023 nbsp 0183 32 In this article we will learn about magnetic quantum numbers their formula and steps to find out magnetic quantum numbers along with their uses
The magnetic quantum number determines the total number of orbitals in a subshell as well as their orientation The symbol ml is used to represent it This value represents the projection of the orbital angular momentum along a specific axis