Corrective And Preventive Action Capa Process Feb 26 2025 nbsp 0183 32 Corrective Action CA Actions to eliminate existing defect to prevent it from happening again Preventive Action PA Actions to prevent potential defects Future problem before they occur Together both actions
Sep 2 2024 nbsp 0183 32 In this article you will learn CAPA CA corrective action PA preventive action the difference between CA and PA case studies the key aspects of CAPA applications Sep 21 2023 nbsp 0183 32 Gain a deep understanding of Corrective and Preventive Actions CAPA in quality management Learn how CAPA processes can drive continuous improvement and compliance
Corrective And Preventive Action Capa Process
 Corrective And Preventive Action Capa Process
Corrective And Preventive Action Capa Process
https://www.onqsoft.com.au/wp-content/uploads/2023/02/Steps-CAPA.png
Jul 12 2024 nbsp 0183 32 The corrective and preventive action CAPA is a structured approach that enables organizations to identify analyze and address nonconformities deviations and potential risks
Templates are pre-designed files or files that can be used for various purposes. They can save effort and time by offering a ready-made format and layout for creating different kinds of material. Templates can be utilized for individual or expert tasks, such as resumes, invitations, flyers, newsletters, reports, discussions, and more.
Corrective And Preventive Action Capa Process
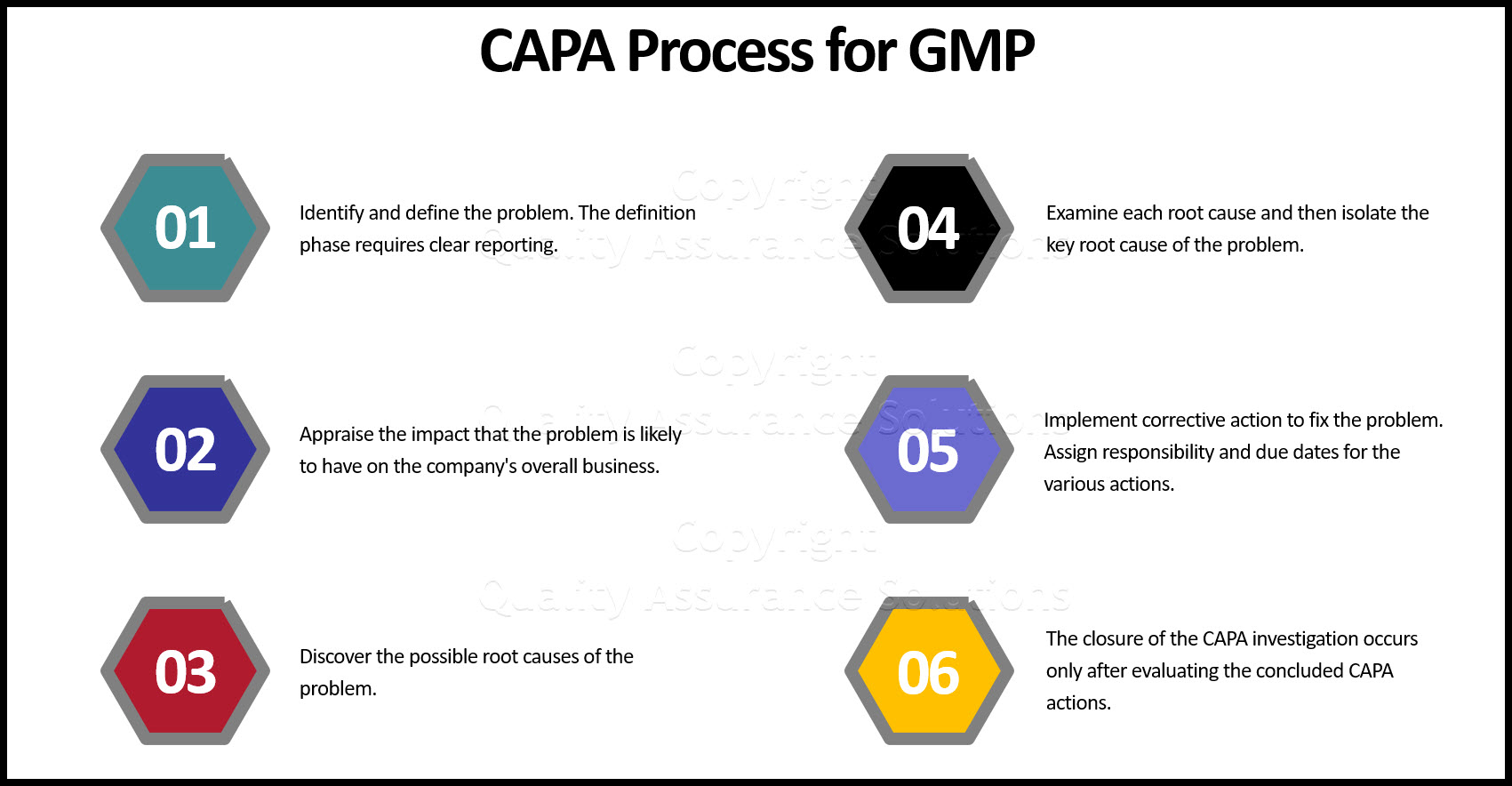
Corrective Action Process
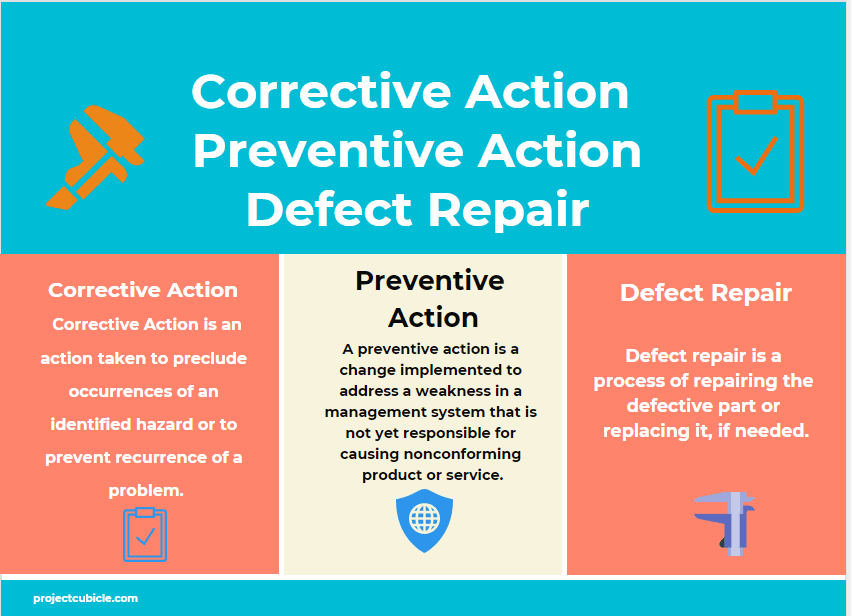
Corrective Action Examples
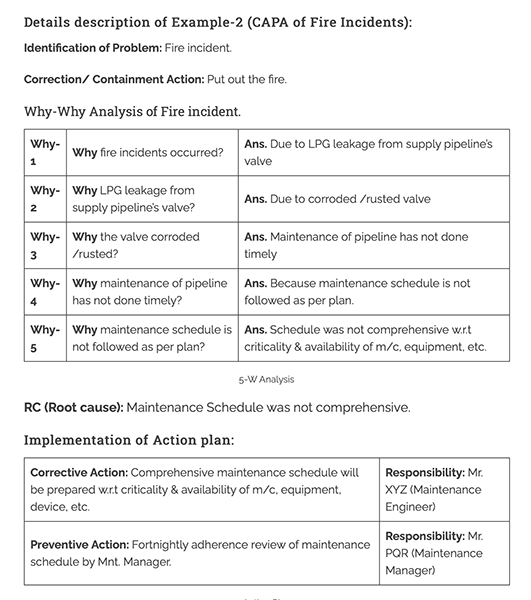
Corrective And Preventive Action CAPA Definition Purpose And
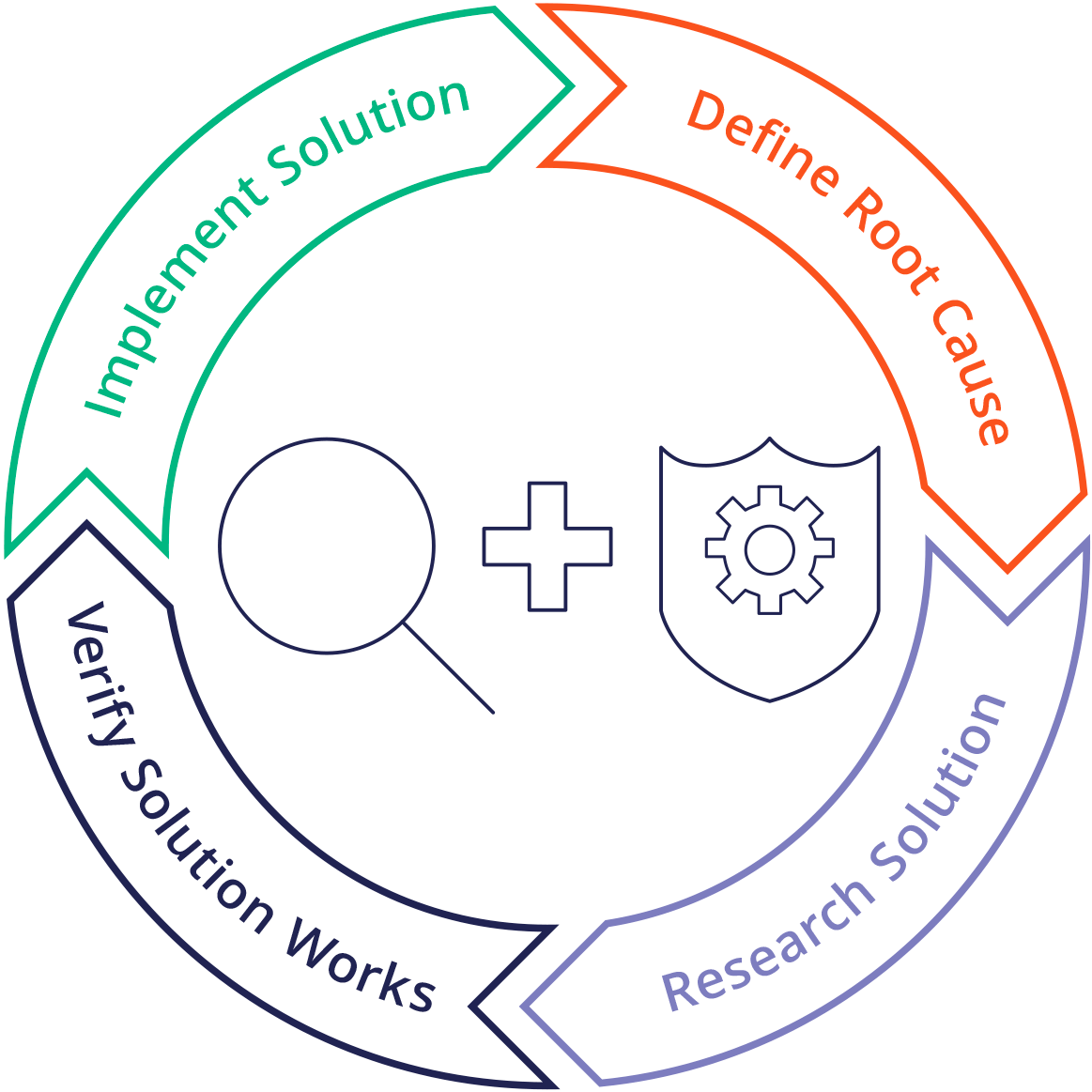
Corrective And Preventive Action CAPA Definition Purpose And
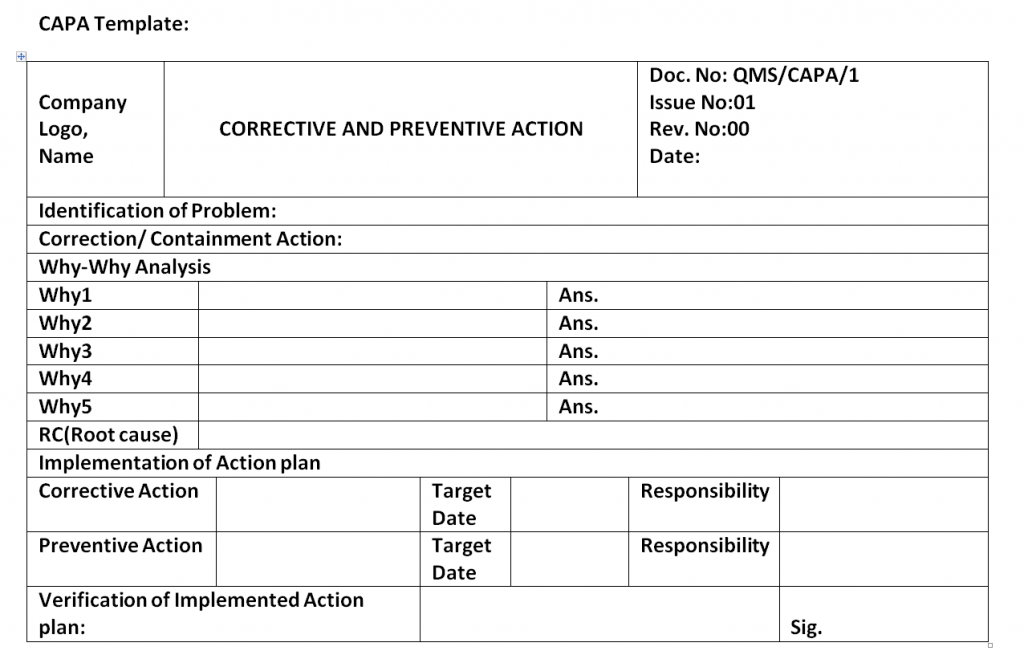
Iso 9001 Corrective Action Examples Gogolasopa

CAPA Form Corrective Action And Preventive Action

https://leanoutsidethebox.com › capa
Apr 29 2025 nbsp 0183 32 What is CAPA CAPA is a structured method for identifying correcting and preventing quality issues It helps teams get to the root cause of problems instead of treating
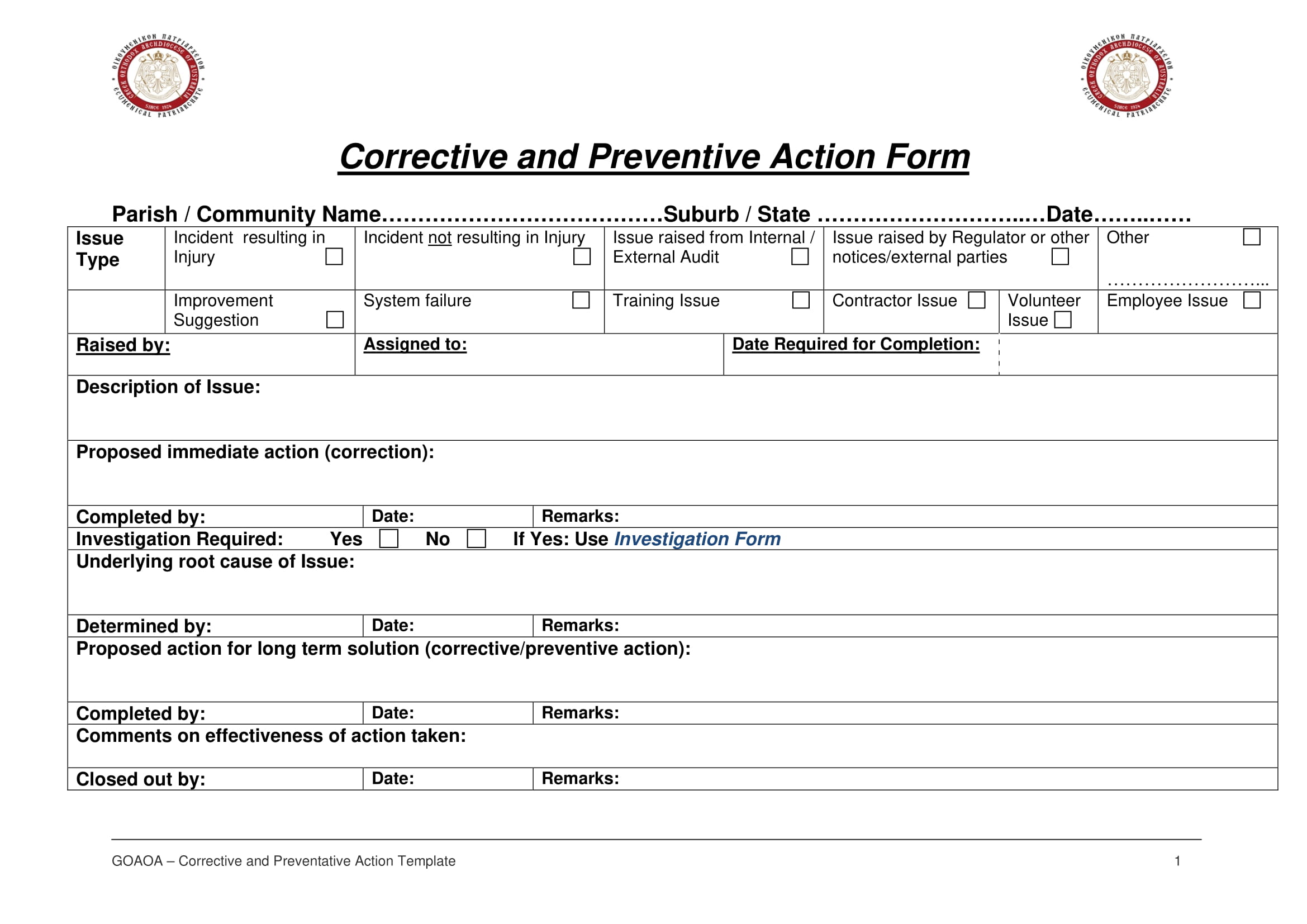
https://www.thefdagroup.com › blog › definitive-guide...
Jan 6 2025 nbsp 0183 32 The purpose of corrective and preventive action is to collect and analyze information identify and investigate product and quality problems and take appropriate and effective corrective and or preventive action to prevent

https://pharmabeginers.com › sop-for-corrective-and...
Mar 16 2020 nbsp 0183 32 Corrective and Preventive Action CAPA is a concept with current Good Manufacturing Practice cGMP that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence corrective

https://simplerqms.com › capa-procedure
Apr 4 2025 nbsp 0183 32 A well structured CAPA procedure helps detect problems conduct root cause analysis implement corrective and preventive actions and verify their effectiveness to prevent recurrence

https://adaptivegrc.com › resources › articles › what...
Jan 25 2024 nbsp 0183 32 The CAPA Corrective and Preventive Action process is a crucial component of any quality management system It involves identifying investigating and addressing issues or nonconformities to prevent their
Apr 13 2025 nbsp 0183 32 Learn what CAPA means in quality management and how it ensures compliance and continuous improvement Discover the definition of CAPA its process Nov 8 2021 nbsp 0183 32 What is Corrective and Preventive Action Corrective and Preventive Action sometimes referred to as CAPA is a quality management strategy that is made up of
Jun 16 2024 nbsp 0183 32 What is the difference between corrective action and preventive action Corrective action is taken to address an existing problem or nonconformance focusing on resolving the